Abstract
Aging profoundly impacts liver physiology by disrupting autophagy, a lysosome-dependent degradation pathway essential for maintaining cellular homeostasis. Autophagy declines with aging due to reduced expression of core autophagy-related (ATG) genes/proteins, defective autophagosome fusion, and impaired selective processes such as lipophagy, mitophagy, and chaperone-mediated autophagy. These alterations contribute to lipid accumulation, oxidative stress, inflammation, and mitochondrial dysfunction, thereby accelerating age-related liver diseases including metabolic-associated fatty liver disease (MAFLD), fibrosis, and hepatocellular carcinoma (HCC). Their molecular mechanisms involve deregulation of nutrient-sensing pathways (mTOR complex 1, AMP-activated protein kinase and sirtuin 1 and 3) and context-dependent roles of autophagy-related proteins (ATG5, ATG7, LC3, Beclin-1, LAMP2A). Importantly, the regulatory role of autophagy differs across disease stages related to liver aging. During early phases, it maintains metabolic balance, mitochondrial quality control, and genomic stability in some diseases such as MAFLD and liver fibrosis. Conversely, in advanced disease, particularly in HCC, persistent autophagy supports tumor cell survival, stemness, and immune evasion. Emerging therapies seek to restore autophagic flux through caloric restriction, physical exercise, caloric restriction mimetics (rapalogs, spermidine, metformin), and pharmacological modulators such as Tat-BECLIN-1 peptides or RUBICON-targeted approaches. However, translating these therapies into clinical practice remains challenging due to systemic effects, stage-specific responses, and lack of reliable non-invasive biomarkers for monitoring autophagy in humans. Advances in nanoparticle-based delivery, biomarker-guided stratification, and combination therapies with tyrosine kinase inhibitors or immune checkpoint inhibitors may offer promising strategies. Overall, precision modulation of autophagy could serve as a potent geroprotective approach to preserve liver function, delay age-related metabolic deterioration, and prevent progression to fibrosis and cancer. Achieving this goal requires considering disease stage, systemic interactions, and autophagy’s context-dependent duality in aging when implementing these strategies.
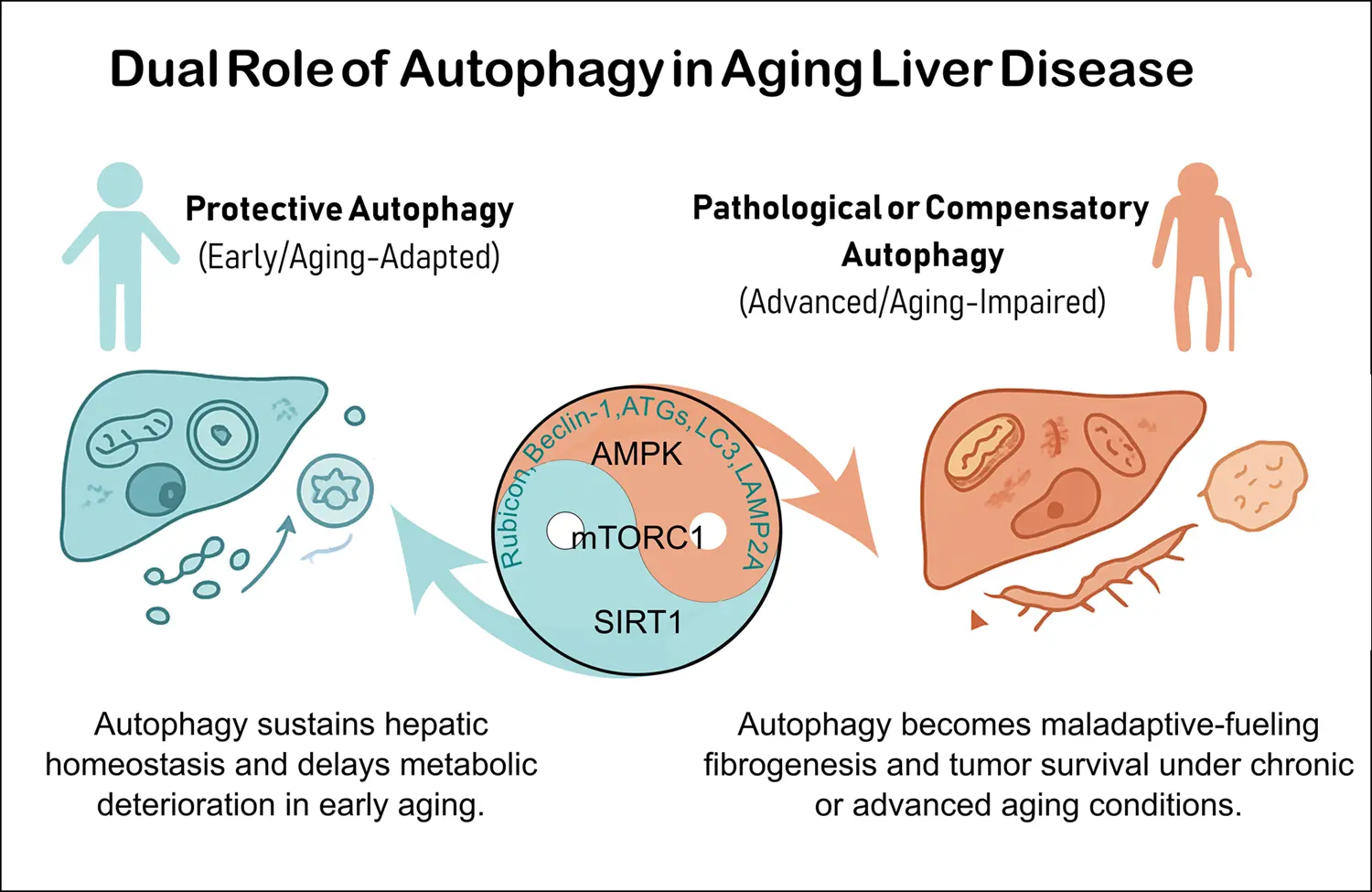
Graphical Abstract
Keywords
1. Introduction
Aging disrupts cellular homeostasis and elevates the risk of chronic diseases, with the liver being particularly vulnerable owing to its central role in metabolic regulation. Consequently, age-related hepatic conditions, such as metabolic-associated fatty liver disease (MAFLD), fibrosis, and hepatocellular carcinoma (HCC), are more prevalent among older populations[1,2].
Autophagy, a conserved lysosome-dependent degradation pathway, maintains hepatic homeostasis by eliminating damaged organelles, misfolded proteins, and lipids[3,4]. With aging, autophagy declines primarily attributed to three core mechanisms: reduced expression of autophagy-related (ATG) core genes/proteins, defective autophagosome-lysosome fusion, and impaired selective processes such as lipophagy, mitophagy, and chaperone-mediated autophagy[4,5]. These alterations collectively drive the onset and progression of multiple age-related liver diseases such as MAFLD, fibrosis and HCC[6-10].
Autophagy efficiency generally declines with aging, contributing to metabolic and age-related disorders such as liver dysfunction[1,11]. Global deletion of essential ATG genes is lethal in mice, while tissue-specific knockouts cause localized dysfunction and premature aging-like phenotypes. In rodents, levels of ATG and lysosome-associated membrane protein 2A (LAMP2A) of liver typically decrease with age, and recent studies suggest that their variability depends on strain, sex, and hepatic cell type[12,13]. Although direct human evidence remains limited at present, impaired autophagy is consistently observed in age-associated liver diseases such as MAFLD and hepatic fibrosis[14,15].
In recent decades, numerous studies have highlighted autophagy restoration as a sort of promising therapeutic strategies (lifestyle interventions and pharmacological modulators) for the aging liver in both preclinical and clinical settings[16]. Although autophagy is disabled in aging[17], its role remains complex and sometimes controversial. Therefore, when considering autophagy modulation in geromedicine, systemic effects, dosing, and comorbidities requires to be carefully considered[18]. The major controversies include the context-dependent nature of autophagy, contradictory findings in loss-of-function models (autophagy-related gene 5 (ATG5), autophagy-related gene 7 (ATG7), Beclin-1), and its dual role of acting as a protective mechanism in early liver disease but potentially promoting tumor progression in advanced HCC.
In this mini review, we summarized current perceptions on autophagy’s decline in geriatric liver, its contribution to age-related liver diseases, and emerging therapeutic strategies aimed at modulating this pathway to preserve liver function of the aged.
2. Autophagy in the Liver
2.1 Basic concepts
Autophagy is a highly selective cellular recycling process that degrades cytosolic proteins and organelles in eukaryotes, and its activity declines with age. Since the early 2000s, research on autophagy has expanded rapidly[1,3].
There are 3 types of autophagy:
• Macroautophagy involves selective and non-selective cytosolic components enclosed in a double membrane-bound vesicle called autophagosome, which subsequently fuses with the lysosome to form an autolysosome[19].
• Microautophagy also implies that selective and non-selective cytosolic components are directly engulfed by the lysosome through membrane invagination[19].
• Chaperone-mediated autophagy (CMA) is a selective process in which soluble proteins with pentapeptide motif for CMA recognition (KFERQ-like motifs) are translocated into lysosomes via LAMP2A[3,19].
2.2 Autophagy role in liver physiology: lipid metabolism, organelle turnover and detoxification
Autophagy plays a central role in maintaining hepatic homeostasis by regulating nutrient metabolism, organelle turnover, and cellular detoxification through both selective and nonselective mechanisms. Under nutrient deprivation, liver autophagy is primarily induced by mTOR complex 1 (mTORC1) inhibition and AMP-activated protein kinase (AMPK) activation. This induction generates essential metabolic substrates including amino acids, glucose, and free fatty acids (FFAs) by intracellular catabolism of proteins, glycogen (glycophagy), and lipid droplets (lipophagy), respectively, thereby supporting global energy balance[19,20].
Regarding lipids metabolism, selective forms of autophagy such as lipophagy prevent hepatic steatosis by generating FFAs for mitochondrial β-oxidation. Lipophagy is a tightly regulated process that involves ubiquitin-mediated cargo tagging and recruitment of adaptor proteins, including sequestosome 1 (SQSTM1), neighbor of BRCA1 gene 1(NBR1) and microtubule-associated protein 1 light chain 3 (LC3)/GABA type A receptor-associated protein (GABARAP)-phagophores. To facilitate lipid transport, lipophagy targets lipid droplet-associated proteins, specifically perilipin 2 (PLIN2) and perilipin 3 (PLIN3)[21]. Besides, hepatic transcription factors (transcription factor EB and transcription factor E3) enhance autophagic flux via lysosome-associated membrane protein 1 and reduce lipid accumulation[22]. Additionally, autophagy promotes mitochondrial β-oxidation by removing inhibitors of peroxisome proliferator-activated receptor alpha, such as nuclear receptor corepressor 1[23].
Autophagy also contributes to intracellular quality control mediated by turnover of selective mitochondria (mitophagy), peroxisomes (pexophagy), and the endoplasmic reticulum (ER-phagy), thus preventing hepatocellular injury. For instance, hepatic mitophagy eliminates dysfunctional mitochondria and mutated mitochondrial DNA through PTEN-induced kinase 1 (PINK1)/Parkin and LC3/mitophagy receptor/BNIP3-like protein complexes, limiting reactive oxygen species (ROS) production and inflammatory signaling[24]. Similarly, ER-phagy, mediated by family with sequence similarity 134 member B, SEC62 homolog (ER-phagy receptor), and testis-expressed protein 264 (ER-phagy receptor) receptors alleviate ER stress in an LC3-dependent manner[25]. Furthermore, pexophagy regulates peroxisome number and function via ataxia telangiectasia mutated (ATM), SQSTM1/peroxisomal biogenesis factor 5, and LC3-dependent mechanism[26].
Additionally, autophagy modulates hepatic detoxification by regulating redox balance and cytochrome P450 enzyme expression. Under different stress conditions, autophagy is upregulated to influence biotransformation, antioxidant responses, and hepatocyte survival. The SQSTM1-nuclear factor erythroid 2-related factor 2 (NRF2) axis illustrates how selective autophagy enhances cytoprotective gene expression and detoxification by stabilizing NRF2[27,28]. Moreover, autophagy activation in response to cellular stressor (lipotoxicity or ER stress) reduces ROS accumulation, preserves organelle function, and limits the activation of Kupffer and hepatic stellate cells (HSCs), protecting against inflammation, fibrosis, and disease progression[29].
Altogether, these selective forms of autophagy not only maintain hepatic homeostasis but also critically modulate the progression of liver diseases. Impaired lipophagy leads to triglyceride accumulation and steatosis[30,31], while defective mitophagy favors ROS production, hepatocyte death, and fibrosis[24]. Likewise, dysregulation of ER-phagy and pexophagy exacerbates ER stress and oxidative injury, contributing to inflammation and fibrogenesis[32,33]. Thus, selective autophagy pathways are tightly linked to the pathogenesis of MAFLD, fibrosis, and even hepatocarcinogenesis, underscoring their dual role as both protective and potentially pathogenic mechanisms depending on the disease context.
3. Age-Related Changes in Hepatic Autophagy
It is known that autophagy efficiency declines with aging, sequentially contributing to liver disorders (Table 1). Nevertheless, recent evidence suggests that the role of autophagy in aging is multifaceted and, at times, controversial[18]. This section reviews age-related changes in autophagy, focusing on molecular regulation, mitochondrial dysfunction, nutrient-sensing pathways and emerging interactions with the gut microbiota.
| Age-related Liver disorder | Target gene/protein | Experimental approach | Autophagy pathway involved | Phenotypic effect | Disease stage/context | Therapeutic strategy tested | References |
| MAFLD | RUBICON (overexpression) | HFD, HCV infection | Macroautophagy | Lipid accumulation, apoptosis, inflammation | Steatosis progression | — | [99,105,164] |
| MAFLD | RUBICON deletion (hepatocyte-specific) | Mouse models | Lipophagy | Reversal of steatosis, ↓ inflammation | Diet-induced MAFLD | siRNA nanoliposomes | [100,123] |
| MAFLD | RUBICON deficiency | Mouse models | Lipophagy | ↑ steatosis (↑ NEFA influx) | Systemic metabolism | — | [106,107] |
| MAFLD | METTL3 → Rubicon mRNA | Mouse models | Macroautophagy | Suppressed autophagy → lipid accumulation | Steatosis | Epitranscripto mic modulation | [104] |
| MAFLD | Tat-BECLIN-1 peptide | Mouse models | Macroautophagy | Improved lipid metabolism, ↓ steatosis | Diet-induced MAFLD | Peptide therapy | [52] |
| Fibrosis | Autophagy in HSCs | HSCs in vitro, mouse fibrosis | Lipophagy | Energy for collagen secretion, fibrosis promotion | Early fibrosis | TGF-β inhibitors | [108-109] |
| Fibrosis | Autophagy in HSCs | HSC regression models | Macroautophagy | HSC quiescence, ↓ collagen | Regression fibrosis | miR-29 mimics, GATA4 induction | [110,113] |
| Fibrosis | ECM stiffening | Human, mouse models | Macroautophagy | Profibrotic activation | Chronic fibrosis | LOXL2 inhibitors (mechanotherap y) | [165] |
| Fibrosis | CD4+ T / macrophages | Human, mouse model | Macroautophagy | ↑ inflammation, HSC activation, fibrosis | Fibrogenic | — | [37,48] |
| HCC | BECLIN-1 and ATG5 (↓ expression) | Human Mouse models | Macroautophagy | ↓ tumor suppression, ↑ HCC risk | Premalignant, cirrhosis | — | [117] |
| HCC | ATG5 (↑ in tumors) | Human | Macroautophagy | Poor differentiation, metastasis, ↓ survival | Advanced HCC | — | [116] |
| HCC | ATG5/7 deletion (hepatocyte-specific) | Mouse models | Macroautophagy | Inflammation, fibrosis, NRF2 activation | Pre-malignant HCC | [118] | |
| Aging liver | ATG5 expression | Mouse models | Macroautophagy | ↑ lifespan, improved aging markers | General aging | — | [19,34] |
| Aging liver | ATG7 decline | Mouse models | Macroautophagy, mitophagy | Steatosis, fibrosis, defective mtDNA clearance | Aging liver | mTOR inhibition (partial) | [41-44] |
| Aging liver | ATG7 rare variants | Human | Macroautophagy | Severe MAFLD, fibrosis, HCC | Human aging | — | [39-40] |
| Aging liver | LC3 decline | Aged hepatocytes | Lipophagy | ↓ autophagosomes, steatosis | Aging liver | — | [13,46-48] |
| Aging liver | Beclin-1F121A mutation | Mouse models | Macroautophagy | ↑ basal autophagy, lifespan extension; overactivation → fibrosis | Aging liver | — | [51-52] |
| Aging liver | LAMP2A decline | Aged mouse models | CMA | Impaired lysosomal fusion, TG accumulation | Aging liver | — | [5,23,53-54] |
| Aging liver | PINK1, Parkin loss | Mouse models | Mitophagy | Dysfunctional mitochondria, ↑ ROS, senescence, steatosis | Aging liver | — | [61-62] |
| Aging liver | SIRT1 decline | Mouse models | Macroautophagy | Impaired autophagosome maturation, ↑ inflammasome | Aging, | SIRT1 activators | [68-69,88-89,47-49] |
| Aging liver | SIRT3 loss | Mouse models | Mitophagy | Hyperacetylation, defective autophagy | Aging | — | [65,67] |
| Hepatotoxicity | — | In vitro (HepG2 cells) | Macroautophagy, mitophagy | ↓ Damage-associated hepatotoxic stress by APAP | Aging-associated hepatotoxic stress | Derived-Postbiotic supplementation (L.fermentum, HV110) | [94] |
| Hepatotoxicity | ATG5 (↑ expression) | Mouse models | Macroautophagy | ↓ Damage-associated hepatotoxic stress by D-Galactose | Aging-associated hepatotoxic stress | Probiotic supplementation (L. plantarum NJAU-01) | [95] |
| Aging liver | ATG5, ULK1, BECLIN-1 (↑ expression) | Mouse models | Macroautophagy | ↓ Steatosis; microbiota remodeling | Diet-induced MAFLD | Apple Polyphenol Extract supplementation | [96] |
| Aging liver | — | Mouse models | Macroautophagy (indirect effects) | ↑ anti-aging metabolites (liver and systemic Spermidine) | General aging | Pro/Postbiotic supplementation (A. muciniphila) | [98] |
APAP: acetaminophen; ATG5: autophagy-related gene 5; ATG7: autophagy-related gene 7; BECN1: beclin-1; CD4: cluster of differentiation 4; CMA: chaperone-mediated autophagy; ECM: extracellular matrix; GATA4: GATA binding protein 4; HCC: hepatocellular carcinoma; HCQ: hydroxychloroquine; HCV: hepatitis C virus; HFD: high-fat diet; HSC: hepatic stellate cell; HV110: Lactobacillus fermentum BGHV110; LAMP2A: lysosome-associated membrane protein 2A; LC3: microtubule-associated protein 1 light chain 3; LOXL2: lysyl oxidase-like 2; MAFLD: metabolic-associated fatty liver disease; METTL3: methyltransferase-like 3; NEFA: non-esterified fatty acids; NRF2: nuclear factor erythroid 2-related factor 2; PINK1: PTEN-induced kinase 1; ROS: reactive oxygen species; SIRT1: sirtuin 1; SIRT3: sirtuin 3; TG: triglycerides; TGF: transforming growth factor β1; ULK1: unc-51 like autophagy activating kinase 1; ↑: increased;↓: decreased.
3.1 Molecular evidence of impaired autophagy in the aged liver
Aging impairs autophagic function in hepatocytes by transcriptionally downregulating autophagy-related genes like Atg5, Atg7, Beclin-1, LC3, and LAMP2A[15]. Pyo and colleagues demonstrated that Atg5 deficiency in hepatocytes promotes hepatic inflammation[34]. In fact, moderate Atg5 overexpression enhances autophagic flux in liver, improves systemic aging markers, and extends lifespan, though liver-specific benefits appear to be context-dependent[34]. In aged mice, macrophage/dendritic-specific Atg5 deletion also skews polarization toward a pro-inflammatory phenotype, aggravating tissue injury[35,36], while T cell-specific deletion exacerbates fibrosis[37]. Conversely, the injury of hepatocyte-targeted Atg5 knockout (KO) mice is mitigated by defective autophagic flux, sustained activation of NRF2 and compensatory proliferation[38].
Declining Atg7 expression in aged hepatocytes not only diminishes autophagic activity but also impairs mitochondrial DNA clearance, compromising quality control. Baselli and other authors have described that rare human Atg7 variants (p.P426L, p.V471A) correlate with severe MAFLD, fibrosis, and HCC progression[39,40]. In mice, Atg7 ablation exacerbates MAFLD induced by high-fat diet (HFD)[41,42]. Accordingly, hepatocyte-specific Atg7 deletion in vivo causes acute hepatitis, defective regeneration, fibrosis, and disrupted lipid metabolism[43,44]. Some of them are partially reverted by mTOR inhibition and afterwards the survival rates can be enhanced[43]. Recent controversial results demonstrated that both systemic and liver-specific Atg7 ablation protects against asparaginase-induced hepatic steatosis[45]. This can be explained by the distinct pathophysiological contexts and metabolic demands. Collectively, these results highlight the dual and context-dependent role of Atg7 in liver physiology, underscoring the need for further studies to clarify its precise contribution.
Alterations in Atg8 (LC3) system are key features of hepatic aging. With aging, LC3B lipidation, autophagosome formation, and lipophagy are reduced[4]. Unlike extrahepatic tissues, the liver fails to maintain LC3-dependent flux[46]. Single-cell data demonstrate more prominent downregulation of Atg8 in hepatocytes than in Kupffer or HSC cells, and further modulation by inflammation and cellular shifts[13,47]. Of note, LC3B-II alone is an imperfect marker, as non-canonical functions such as LC3-associated phagocytosis remain active, exerting anti-inflammatory and anti-fibrotic effects, whereas their inhibition worsens fibrosis[48]. Another non-canonical pathway, Atg8ylation described by Deretic and Lazarou, involves the covalent attachment of Atg8 proteins to membranes and is altered with aging[49,50], but its role in the liver remains unclear.
Modulation of Beclin-1 yields mixed benefits. Disruption of the Beclin-1-BCL2 interaction (Becn1F121A/F121A mice) and beclin-1 peptides in vivo enhance hepatic autophagy and extends lifespan[51,52].However, aged hepatocytes fail to mount sufficient Beclin-1-driven responses to protect against age-related liver phenotype[46]. Thus, Beclin-1 plays a pivotal role in triggering autophagy in the aging livers.
Additionally, Cuervo and others have demonstrated that age-related LAMP2A loss impairs autophagosome-lysosome fusion and CMA, reducing fatty acid oxidation and promoting lipid accumulation[5,23,53]. This decline is more pronounced in aged males than in females[54], but it is not universal, as CMA activity can remain stable or increase in certain genetic backgrounds (UM-HET3 and C57BL/6J, respectively)[55,56]. Recent studies also indicate sex-specific differences in hepatic autophagic responses under metabolic stress: female rats display impaired autophagic flux (mediated by LAMP2A) and reduced antioxidant activation compared to males[57]. Notably, in mice overexpressing specific cytochrome CYB5R3, females, but not males, exhibit enhanced mitochondrial turnover (mitophagy) and differential regulation of autophagy markers, further highlighting the sex-dependent nature of autophagic adaptation[58]. These findings suggest that CMA vulnerability to aging is context-dependent, shaped by genetic and endocrine factors, thereby complicating the development of broadly applicable autophagy-targeted therapies.
Finally, it is important to emphasize that all of the above-mentioned alterations in ATG5, ATG7, Beclin-1, LC3, and LAMP2A are not isolated events, but occur within a collaborative regulatory network. Autophagosome biogenesis is initiated by the assembly of a Beclin-1-dependent complex, which recruits the ATG5-ATG12-ATG16L1 conjugation system and ATG7 to promote LC3 lipidation and membrane expansion, culminating in LAMP2A-mediated lysosomal fusion. Consequently, age-related decline in any single component can disrupt the entire cascade, amplifying defects in autophagic flux and exacerbating liver aging phenotypes[59,60].
3.2 Mitochondrial dysfunction in the aging liver
Mitochondrial quality maintained through mitophagy, but declines with aging. In the liver, mitochondrial impairment is characterized by reduced mitophagy flux, which disturbs redox homeostasis due to the accumulation of depolarized mitochondria and excessive ROS production[61]. Under this stress condition, key regulators such as PINK1, Parkin, BNIP3, and sirtuin 1 (SIRT1) become dysfunctional, thereby exacerbating the liver injury[62]. Nonetheless, Parkin-independent, p62/SQSTM1-mediated hepatic mitophagy remains partially active, providing a compensatory mechanism that delays mitochondrial collapse[63]. Liver-specific Atg7 knockout models underscore the role of basal autophagy in preserving mitochondrial DNA integrity[64].
Mitochondrial dysfunction also reflects impaired dynamics in the aged liver. One common process is the hyperacetylation of fusion/fission proteins (MFN2, PINK1) as reduced SIRT1 and SIRT3 activity disturb morphology and ATP production[65,66]. SIRT3 loss, in combination with ATG4B deficiency, further compromises autophagosome maturation[67]. Moreover, increased ROS production associated with hepatic SIRT1 loss induces bioenergetic failure and proinflammatory responses, including activation of the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome and ER stress, thereby promoting fibrosis and inflammmaging[68,69]. Importantly, mitochondrial autophagy alterations are not limited to hepatocytes, but also involve endothelial dysfunction, loss of zonation, and HSCs cell activation, highlighting the multicellular nature of hepatic aging[10,70].
3.3 Nutrient sensing and autophagy in the aging liver
Physiological decline associated with aging, including insulin resistance, hormonal imbalance, and chronic low-grade inflammation, markedly influences the metabolism of autophagy regulators. These systemic alterations impair nutrient-sensing pathways such as mTOR, AMPK, and SIRT1, diminishing their ability to properly respond to metabolic cues. Consequently, the progressive changes of these pathways contribute to the heterogeneous decline of autophagic activity observed among elderly populations[1,68,71-73].
The mTOR, AMPK, and SIRT1 pathways that depend on nutrients status can impact autophagy in the aged liver[74]. Mild chronic activation of mTORC1 reduces lifespan by approximately 30% and promotes parenchymal injury, inflammation, and senescence features in mice[71], while dysregulated nutrient and hormonal signaling to mTORC1 alters hepatocyte metabolic zonation[72]. Persistent mTORC1 hyperactivation suppresses unc-51 like autophagy activating kinase 1 (ULK1)-mediated initiation and lysosomal gene transcription, driving accumulation of damaged organelles[75,76]. Although pharmacological mTOR inhibition restores liver autophagy[77,78], it may impair regenerative capacity in vivo[79,80], suggesting the need for mTOR-independent strategies such as AMPK-ULK1 activation without compromising regenerative capacity[81].
AMPK, traditionally viewed as a pro-autophagic and longevity-promoting kinase[82], supports mitochondrial[83] and lysosomal function[84]. However, Park et al. indicated that under energy stress, hepatic AMPK can paradoxically inhibit autophagy by suppressing ULK1 activity, contrary to its canonical function[85]. Simultaneously it can protect ULK1 and the associated autophagy machinery from caspase-mediated degradation, thus preserving the capacity to resume autophagy upon recovery in vivo[86]. This dual regulatory capacity suggests that AMPK functions both as a brake, preventing excessive autophagy activation, and as a safeguard, preserving the integrity of its core components.
SIRT1 can control autophagy by deacetylating ATG proteins and transcription factors regulating lysosomal function. Its age-related decline impairs autophagosome maturation, aggravates mitochondrial dysfunction, and enhances inflammasome activation[69,87]. Nutrient-induced O-linked β-N-acetylglucosaminylation (post-translational modification) activates SIRT1, protecting hepatic cells from stress[88]. Similarly, SIRT1 restoration in HFD-treated mice attenuates liver lipotoxicity[88,89]. Nonetheless, in hepatocarcinogenesis, SIRT1 activation under glucose deprivation can reprogram metabolism[90] or promote tumorigenic pathways via p62/SQSTM1[91], contributing to liver cancer progression via autophagy-dependent[92,93] or autophagy-independent pathway[91], respectively. Thus, SIRT1 exemplifies the duality of nutrient-sensing pathways in the aging liver, acting as both a guardian of metabolic homeostasis and, under pathological conditions, a driver of disease progression.
3.4 Microbiota and autophagy in liver aging
The gut microbiota exerts a profound influence on hepatic metabolism and aging, partly by modulating autophagic pathways. Recent studies have explored the interplay between microbial products, autophagy, and liver protection. For instance, Lactobacilli, a genus of beneficial bacterium commonly found in fermented foods, has been shown to modulate autophagy in hepatocytes. A postbiotic compound derived from Lactobacillus fermentum BGHV110 (HV110) activates autophagy in HepG2 cells and protects against acetaminophen-induced toxicity[94]. Similarly, supplementation with Lactobacillus plantarum NJAU-01 protects mouse liver from D-galactose-induced oxidative stress and aging-related damage[95].
Polyphenol-rich dietary components can also modulate the gut-liver axis. For example, apple polyphenol extract (APE) protects the liver of aged mice from HFD-induced damage, promoting autophagy and increasing the Firmicutes/Bacteroidetes ratio and Akkermansia abundance[96]. However, the mechanistic link between APE-induced autophagy and microbiota remodeling remains unclear.
Studies in mice with defective hepatic autophagy have revealed that impaired autophagic flux is associated with altered bile acid metabolism, which in turn leads to adaptive changes in microbiota composition[97]. Interestingly, pasteurized Akkermansia muciniphila increases the hepatic levels of anti-aging metabolites such as N1, N12-diacetylspermine[98], suggesting that modulation of the microbiota may indirectly influence liver aging through metabolite-mediated autophagy regulation.
In summary, although emerging evidence links microbiota-derived factors and autophagy to hepatoprotection during aging, the underlying causal and mechanistic relationships remain poorly understood. Further studies are needed to elucidate whether microbiota-induced autophagy activation represents a viable strategy for preventing or reversing age-related hepatic decline.
4. Implications for Age-Related Liver Disease and Autophagy
Autophagy dysfunction is known to contribute to disease onset and progression of various age-related liver pathologies (Table 1). Reduced ATG gene expression limits the capacity to remove misfolded proteins, promoting aggregate accumulation in MAFLD and fibrosis. Defective fusion exacerbates oxidative stress and mitochondrial dysfunction, accelerating hepatocyte death in MALFD and fibrosis. Additionally, impaired selective autophagy drives lipid accumulation, a hallmark of MAFLD that predisposes to inflammation, fibrosis, and ultimately HCC[6-10].
4.1 Autophagy and MAFLD
The aging liver undergoes progressive decline of autophagy, with lipophagy that is the selective degradation of lipid droplets being particularly compromised. This dysfunction directly contributes to the development of MAFLD. A central regulator of this process is Rubicon, an autophagy inhibitor that impairs autophagosome-lysosome fusion and accumulates in senescent hepatocytes under metabolic stress such as high-fat diets or HCV infection. Rubicon overexpression exacerbates lipid accumulation, apoptosis, and inflammation, whereas hepatocyte-specific deletion reverses steatosis in preclinical models, underscoring its therapeutic relevance[99,100]. Mechanistically, Rubicon inhibition enhances lipid flux not only by relieving the autophagosome-lysosome fusion blockade but also by restoring proper lysosomal trafficking of lipid droplet cargo. Loss of Rubicon enables SNARE-mediated vesicle tethering, allowing LC3-decorated autophagosomes to efficiently fuse with cathepsin-active lysosomes[101]. Within this compartment, lysosomal acid lipase hydrolyzes triglycerides into fatty acids, which are subsequently directed toward β-oxidation rather than cytosolic re-esterification[102]. Importantly, hepatic lipophagy induction reduces lipid accumulation even in ATGL-knockout livers, indicating an ATGL-independent pathway. This lipid clearance occurs without detectable increases in circulating NEFAs, implying that the enhanced turnover favors oxidative disposal over systemic hyperlipolysis[103].
An additional epitranscriptomic layer has been identified: METTL3-mediated m6A methylation of Rubicon mRNA suppresses autophagic flux, providing novel regulatory mechanisms and therapeutic targets[104]. Viral cofactors exacerbate this dysfunction, as HCV infection enhances Rubicon expression, thereby creating synergy between metabolic and viral insults in aging patients[105]. Recent evidence further indicates that rare loss-of-function variants in ATG7 increase susceptibility to severe fatty liver disease, highlighting the role of autophagy-related gene defects in the MAFLD progression[39].
Emerging hepatocyte-targeted therapies include anti-Rubicon small interfering RNA (siRNA) delivered via nanoparticles, which reduces steatosis and inflammation in diet-induced or FFAs-overload models[99,100], and the pro-autophagic Tat-Beclin-1 peptide, which improves MAFLD phenotypes[52]. However, a paradox has emerged: systemic Rubicon deficiency worsens fasting-induced steatosis due to uncontrolled peripheral lipolysis and increased circulating NEFAs[106]. This underscores the importance of tissue-specific modulation of autophagy regulators, particularly in metabolically vulnerable aging contexts.
This complexity is further compounded by the liver-adipose axis: age-related loss of Rubicon in adipocytes enhances lipolysis, increasing hepatic fatty acid influx and accelerating lipotoxicity[107]. Therefore, effective MAFLD management in aging individuals necessitates a dual vigilance-restoring hepatocyte-specific autophagy while carefully monitoring systemic lipid flux to prevent maladaptive lipolytic cascades.
4.2 Autophagy and liver fibrosis
Hepatic stellate cells display a context-dependent dual role of autophagy during fibrosis, with disease stage determining whether autophagy promotes or restrains pathology. During early activation, autophagy facilitates the transition to collagen-producing myofibroblasts by mobilizing retinoid and lipid stores to meet energy. This process is reinforced by TGF-β/Smad signaling and mechanosensitive responses to extracellular matrix (ECM) stiffness[108]. At this stage, pharmacologic inhibition of autophagy-related pathways, such as mTOR activation or ATG5 silencing, selectively disrupts lipid catabolism and impedes myofibroblast differentiation, underscoring the therapeutic potential of targeting autophagic fluxin early fibrosis[109].
In contrast, during regression, autophagy promotes HSC deactivation by releasing extracellular vesicles enriched with miR-29, which suppress collagen synthesis and induce quiescence[110]. In this case, therapeutic interventions, including activating GATA binding protein 4 (GATA4)/miR-29 and inducing mitophagy, can be employed to enhance selective autophagy, thereby restoring metabolic homeostasis and reinforcing the return to quiescence.
Aging exacerbates fibrogenesis through systemic dysfunction, characterized by impaired autophagy in liver infiltrated CD4+ T cells. This impairment drives IL-17-mediated type 3 inflammation and the release of profibrotic cytokines that sustain HSC activation[37]. Moreover, arsenic-induced fibrosis models demonstrate that autophagy-derived cytosolic cathepsin B activates the NLRP3 inflammasome in HSCs, amplifying fibrogenesis triggered by environmental exposure[111]. This generates a self-sustaining circuit resistant to monotargeted therapies.
Recent therapeutic paradigms emphasize the importance of temporal precision. In early-stage fibrosis, inhibitors of TGF-β1-induced autophagy such as salvianolic acid B attenuate collagen deposition by blocking pro-activation signals[112]. Conversely, during regression, enhancement of GATA4 activity or miR-29 signaling promotes autophagic clearance of profibrotic mediators[113]. Advances in nanoparticle-based delivery systems, enable spatially targeted modulation of autophagy while minimizing off-target effects, which is a critical consideration for polymedicated older patients[114].
Furthermore, age-related ECM stiffening hyperactivates mTOR-dependent profibrotic autophagy in HSCs, suggesting therapeutic synergy between autophagy modulators and mechanotherapy (e.g., lysyl oxidase-like 2 inhibitors)[115]. Collectively, these findings emphasize that effective antifibrotic strategies must integrate disease stage, immune senescence, and biomechanical alterations into the aging hepatic microenvironment.
4.3 Autophagy and HCC
Autophagy exerts temporally bidirectional effects in HCC, requiring distinct therapeutic strategies based on disease stage. In premalignant conditions or early tumorigenesis, autophagy exerts tumor-suppressive functions by clearing damaged organelles, mitigating oxidative stress, and maintaining genomic stability. Clinically, low serum Beclin-1 and altered ATG5 expression in cirrhotic patients correlate with increased HCC risk, highlighting autophagy’s protective role during malignant transformation[116,117]. Consistently, hepatocyte-specific loss of autophagy (e.g., ATG7 or ATG5 deletion) accelerates dedifferentiation toward ductular progenitor-like cells through YAP/TAZ, facilitating malignant initiation[118].
In established tumors, however, autophagy adopts a tumor-promoting role: it recycles nutrients to sustain proliferation, maintains cancer stem cell plasticity by modulating EMT, and facilitates immune evasion through PD-L1 regulation[119]. This is reflected in clinical cohorts, where high ATG5 expression predicts poor differentiation, metastasis, and reduced disease-free survival in advanced HCC[116]. Notably, macrophage-specific autophagy has been shown to restrain hepatocarcinogenesis by limiting PD-L1 expression and preserving antitumor immunity, underscoring the complexity of cell-type-specific functions[120].
Therapeutic translation thus requires stage-specific precision. In high-risk patients (e.g. cirrhosis from MAFLD), autophagy inducers such as rapalogs may prevent tumor initiation by restoring cellular homeostasis[121]. In contrast, in advanced HCC, autophagy inhibitors such as hydroxychloroquine can overcome adaptive resistance to therapy, sensitizing tumors to sorafenib, lenvatinib, and ICIs[122].
The Rubicon-MAFLD/HCC axis introduces a critical dimension: hepatocyte-specific inhibition of Rubicon reduces inflammation and profibrotic remodeling in MASH, potentially lowering HCC risk[123]. However, in established tumors, Rubicon deletion could paradoxically enhance pro-survival autophagic flux, necessitating combination therapies with cytotoxic or anti-angiogenic agents.
Emerging clinical frameworks prioritize biomarker-guided strategies, integrating ATG5/Beclin-1 quantification with dynamic PET imaging of autophagy to stratify patients into tailored regimens of autophagy modulators, TKIs, and ICIs[116,124,125]. In elderly populations who often present with advanced HCC and metabolic comorbidities, therapeutic strategies must balance efficacy with tolerability, emphasizing sequential or low-toxicity approaches tailored to the distinct pathophysiological features of aging.
Taken together, these findings reinforce the concept that autophagy dysfunction represents a critical turning point in liver pathophysiology during aging. In addition to its roles in lipid metabolism and inflammatory regulation, the progressive loss of this pathway impairs the liver’s capacity to manage chronic insults and limits its reparative mechanisms, thereby facilitating the transition toward fibrosis and cancer. Understanding these interactions may help guide therapies that preserve autophagy and slow down age-related liver decline.
5. Therapeutic Modulation of Autophagy
5.1 Autophagy targeted therapies
Autophagy-targeted therapies emerge as promising alternatives for various diseases (Table 2). Several current interventions are known to regulate autophagy. Caloric restriction (CR) and exercise are widely recommended lifestyle modifications to improve health across many disease conditions[126,127]. CR and exercise have proven to be effective in managing hypertension[128,129], metabolic syndrome[130,131] and liver diseases[132]. The two interventions activate multiple mechanisms including autophagy, which may underlie its beneficial effects[133,134].
| Intervention | Evidence | Autophagy activation | Endogenous | Side effects | |
| CR | MASLD[166,167] | Macroautophagy and CMA | - | - | |
| Exercise | MASLD[168,169] | Macroautophagy and CMA | - | - | |
| Tat-beclin1 peptide | Mitigate MASLD | Direct activator of autophagy | Atg6/Beclin1-derived | Accelerate atheroma plate formation in mice[170] | |
| anti-Rubicon siRNA | Mitigate MASLD | ↓Autophagy inhibition | No | Lipodystrophy, infertility and exacerbation of autoimmune diseases[164] | |
| CRM | Sirolimus | Reduced intrahepatic inflammation in MASLD | Inhibit mTORc1 | No | Anemia, thromboembolism, diabetes, dyslipidemia, kidney damage, mucositis and stomatitis, lung problems, angioedema, lymphedema and osteonecrosis[159] |
| Spermidine | Liver fibrosis | Inhibit acetyltransferase EP300 | Yes | Safe profile[158] | |
| Metformin | MASLD in children and adolescents | Activates AMPK | No | Gastrointestinal side effects including diarrhoea, nausea, vomiting, flatulence, abdominal pain, and loss of appetite[160] | |
| Lithocholic acid | ↑ Liver healthspan and lifespan | Activates AMPK | Yes | Rosacea-like skin[171]; ↑ coronary atheroma risk[172] | |
| Acadesine | Improved maternal hepatic lipid metabolism disorder | Activates AMPK | Purine nucleoside analogue | Safe profile[173] | |
MASLD: metabolic dysfunction-associated steatotic liver disease; CR: caloric restriction; CMA: chaperone-mediated autophagy; Atg6: autophagy-related gene 6; siRNA: small interfering RNA; CRM: caloric restriction mimetics; mTORC1: mechanistic target of rapamycin complex 1; AMPK: AMP-activated protein kinase; EP300: E1A binding protein P300; ↑: increased;↓: decreased.
CR stands for a reduction in daily caloric intake while preserving all essential nutritional requirements[127]. It induces CMA by stabilizing LAMP2A levels in the livers of rats, preventing the age-related decline in autophagy which is observed in these animals[3]. It also helps attenuate the decrease in macroautophagy[135]. Exercise, for its part, robustly activates both macroautophagy and CMA in liver and muscle tissues of C57BL/6J mice and human, as evidenced by increased autophagic markers and LC3-II/LC3-I ratios[136,137]. CR mimetics (CRMs) are compounds that emulate the effects of CR and are considered geroprotective molecules. Several substances are classified as CRMs, including rapalogs (sirolimus), spermidine and metformin[3,138]. Since autophagy has been associated with various diseases, and CRMs activate autophagy, these substances possess a potential therapeutic activity[19]. Particularly, an inhibitor of autophagy-suppressive acetyltransferase EP300 spermidine, has proven to be effective for preventing liver MALFD/fibrosis through an autophagy-dependent mechanism in mice treated with CCl4[139] and Western diets[140,141]. Metformin activated by AMPK and sirolimus inhibited by mTORc1, regulate autophagy and are also classified as CRMs. Their combination protects rat erythrocytes against age-dependent oxidative stress[142]. Moreover, both sirolimus[143] and metformin[144,145] have demonstrated protective effects against MASLD in mice and humans, respectively. Another AMPK activator extends hepatic healthspan and lifespan in preclinical and clinical trial[84,146], and acadesine specifically mitigates the hepatic lipid metabolism disorders, inflammation, and fibrosis caused by environmental contamination exposure during pregnancy[147]. More recently, acyl-CoA binding protein/diazepam binding inhibitor has been identified as positively correlated with aging in patients, and its neutralization induces autophagy and produces beneficial effects on aging[148] and liver damage[149-151].
As previously noted, Tat-Beclin-1 peptides[52] and anti-Rubicon siRNA delivered by nanoliposomes[123,100] represent promising autophagy-based strategies for treating age-related liver disease such as MAFLD and fibrosis.
Nevertheless, it is important to highlight that autophagy modulation remains controversial. While autophagy activation shows beneficial effects in aging and metabolic disorders, it may also promote tumor cell survival in cancer, particularly at advanced stages[152,153].
5.2 Targeting autophagy in aged individuals
Given the beneficial effects of autophagy activation on aging-derived problems and other pathologies described in Table 2, targeting autophagy activation appears to be a promising strategy to improve health in elderly populations[154]. Ideally, lifestyle interventions such as CR and exercise could be employed to activate autophagy in older adults with existing health issues or in healthy individuals for preventive purposes. However, these interventions are frequently neglected by patients. Efforts have been made to identify barriers to adopting such changes, including psychological and social factors. Unfortunately, most patients tend to prefer medication over lifestyle changes[155].
In this context, CRMs emerge as good alternatives. Spermidine, a natural polyamine widely present in animals, exhibits decreased plasma levels with aging[156]. It produced beneficial effects on the brain, kidney, liver, cardiovascular system and immune system[156,157]. In a 3-month randomized, placebo-controlled, double-blind phase II clinical trial, elderly patients were supplemented daily with 1.2 mg spermidine derived from wheat extract. The supplementation had produced no effects on vital signs, weight, clinical chemistry parameters, or hematological safety markers, while significantly improving memory performance[158]. On the contrary, sirolimus produces a wide range of side effects, including anemia, thromboembolism, diabetes, dyslipidemia, kidney damage, mucositis, stomatitis, lung problems, angioedema, lymphedema and osteonecrosis[159]. Metformin causes gastrointestinal side effects in up to 30% of patients, including diarrhea, nausea, vomiting, flatulence, abdominal pain, and loss of appetite[160]. Acadesine, an AMPK activator with low specificity, increases uric acid production and favors lactic acidosis[161]. The use of liver-specific, such as AMPK activators, may mitigate these potential side effects[146]. More studies in humans must be carried out to fully explore the therapeutic potential and associated risks of these compounds.
Ultimately, the clinical translation of these interventions remains challenging because there are no reliable, non-invasive biomarkers to monitor autophagy activity in humans. Commonly used markers, such as LC3 and p62, are difficult to interpret outside controlled experimental settings, since they provide only a static picture of autophagy. In fact, the most reliable in vivo approaches require the use of autophagy flux inhibitors, which introduce additional perturbations and limit their applicability in clinical contexts[162,163].
6. Conclusions
Autophagy acts as a central regulator of age-related liver diseases, with key genes and proteins such as RUBICON, BECLIN-1, ATG5/7, and sirtuins shaping disease progression. Dysregulation of lipophagy, mitophagy, and lysosomal fusion contributes to hepatic steatosis, fibrosis, and HCC. Notably, autophagy plays a dual role in cancer, suppressing tumor initiation but supporting tumor growth at advanced stages. Interventions including CR, CRMs, exercise, and pharmacologic modulators demonstrate stage-dependent benefits. However, their clinical translation is hindered by systemic effects, comorbidities, and the lack of reliable biomarkers. Future advances will require liver-targeted strategies and integrative approaches to leverage autophagy as a geroprotective therapy.
Acknowledgments
We acknowledge that not all relevant papers could be included due to space limitations. AI-based software was used to assist with language editing during the preparation of this manuscript. The authors take full responsibility for the content of the manuscript.
Authors contribution
Palacios-Ramírez P, Francés DE: Writing the manuscript, visualization.
Motiñol O: Conceptualization, writing-original draft.
All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
Omar Motiño García-Miguel is an inventor on a patent covering therapeutic targeting of ACBP/DBI.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This study was supported by Proyecto Generación del Conocimiento 2024 (PID2024-161399OA-I00) from Agencia Estatal de Investigación / Ministerio de Ciencia, Innovación y Universidades, Spain; Proyectos de investigación para potenciar el talento y la consolidación de grupos de investigación noveles 2025 (PRONOVUVA2025-07) from Universidad de Valladolid, Spain; Research Grant PICT 2021-0152 from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina; and PIP 621 from CONICET, Argentina. O.M. is supported by the “Beatriz Galindo Junior” Program (BG22/00104) from Ministerio de Ciencia, Innovación y Universidades, Spain.
Copyright
©The Author(s) 2025.
References
-
1. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186(2):243-278.[DOI]
-
2. Wang W, Xu K, Shang M, Li X, Tong X, Liu Z, et al. The biological mechanism and emerging therapeutic interventions of liver aging. Int J Biol Sci. 2024;20(1):280-295.[DOI]
-
3. Jafari M, Macho-González A, Diaz A, Lindenau K, Santiago-Fernández O, Zeng M, et al. Calorie restriction and calorie-restriction mimetics activate chaperone-mediated autophagy. Proc Natl Acad Sci U.S.A. 2024;121(26):e2317945121.[DOI]
-
4. Lim SHY, Hansen M, Kumsta C. Molecular mechanisms of autophagy decline during aging. Cells. 2024;13(16):1364.[DOI]
-
5. Schneider JL, Suh Y, Cuervo AM. Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metab. 2014;20(3):417-432.[DOI]
-
6. Qian H, Chao X, Williams J, Fulte S, Li T, Yang L, et al. Autophagy in liver diseases: A review. Mol Aspects Med. 2021;82:100973.[DOI]
-
7. Hwang JS, Lai TH, Kim DR. Targeting lipophagy in liver diseases: Impact on oxidative stress and steatohepatitis. Antioxidants. 2025;14(8):908.[DOI]
-
8. Mohammed WH, Sulaiman GM, Abomughaid MM, Klionsky DJ, Abu-Alghayth MH. The dual role of autophagy in suppressing and promoting hepatocellular carcinoma. Front Cell Dev Biol. 2024;12:1472574.[DOI]
-
9. Guan L, Guo L, Zhang H, Liu H, Zhou W, Zhai Y, et al. Naringin protects against non‐alcoholic fatty liver disease by promoting autophagic flux and lipophagy. Mol Nutr Food Res. 2023;68(3):2200812.[DOI]
-
10. Chen J, Jian L, Guo Y, Tang C, Huang Z, Gao J. Liver cell mitophagy in metabolic dysfunction-associated steatotic liver disease and liver fibrosis. Antioxidants. 2024;13(6):729.[DOI]
-
11. Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17(11):647-661.[DOI]
-
12. Endicott SJ. Chaperone-mediated autophagy as a modulator of aging and longevity. Front Aging. 2024;5:1509400.[DOI]
-
13. Yang C, Xia S, Zhang W, Shen HM, Wang J. Modulation of Atg genes expression in aged rat liver, brain, and kidney by caloric restriction analyzed via single-nucleus/cell RNA sequencing. Autophagy 2022;19(2):706-715.[DOI]
-
14. González-Rodríguez Á, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014;5(4):e1179.[DOI]
-
15. Le Couteur DG, Ngu MC, Hunt NJ, Brandon AE, Simpson SJ, Cogger VC. Liver, ageing and disease. Nat Rev Gastroenterol Hepatol. 2025;22(10):680-695.[DOI]
-
16. Chaudhary S, Chaudhary MR, Jena MK, Rath PK, Mishra BP, Paital B, et al. Calorie restriction mimetics against aging and inflammation. Biogerontology. 2025;26(4):126.[DOI]
-
17. Kroemer G, Maier AB, Cuervo AM, Gladyshev VN, Ferrucci L, Gorbunova V, et al. From geroscience to precision geromedicine: Understanding and managing aging. Cell. 2025;188(8):2043-2062.[DOI]
-
18. Singh S, Carosi JM, Dang L, Sargeant TJ. Autophagy does not always decline with ageing. Nat Cell Biol. 2025;27(5):712-715.[DOI]
-
19. Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863.[DOI]
-
20. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131-1135.[DOI]
-
21. Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17(6):759-770.[DOI]
-
22. Fang Y, Ji L, Zhu C, Xiao Y, Zhang J, Lu J, et al. Liraglutide alleviates hepatic steatosis by activating the TFEB-regulated autophagy-lysosomal pathway. Front Cell Dev Biol. 2020;8:602574.[DOI]
-
23. Choi YJ, Yun SH, Yu J, Mun Y, Lee W, Park CJ, et al. Chaperone-mediated autophagy dysregulation during aging impairs hepatic fatty acid oxidation via accumulation of NCoR1. Mol Metab. 2023;76:101784.[DOI]
-
24. Undamatla R, Fagunloye OG, Chen J, Edmunds LR, Murali A, Mills A, et al. Reduced mitophagy is an early feature of NAFLD and liver-specific PARKIN knockout hastens the onset of steatosis, inflammation and fibrosis. Sci Rep. 2023;13(1):7575.[DOI]
-
25. Sun Y, Wang X, Yang X, Wang L, Ding J, Wang CC, et al. V-ATPase recruitment to ER exit sites switches COPII-mediated transport to lysosomal degradation. Dev Cell. 2023;58(23):2761-2775.e5.[DOI]
-
26. Zhang J, Tripathi DN, Jing J, Alexander A, Kim J, Powell RT, et al. ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat Cell Biol. 2015;17(10):1259-1269.[DOI]
-
27. Mukherjee R, Bhattacharya A, Mello-Vieira J, Kuncha SK, Hoffmann M, Gonzalez A, et al. Serine ubiquitination of SQSTM1 regulates NFE2L2-dependent redox homeostasis. Autophagy. 2024;21(2):407-423.[DOI]
-
28. Lee DH, Park JS, Lee YS, Han J, Lee DK, Kwon SW, et al. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy. 2020;16(11):1949-1973.[DOI]
-
29. Raza S, Rajak S, Singh R, Zhou J, Sinha RA, Goel A. Cell-type specific role of autophagy in the liver and its implications in non-alcoholic fatty liver disease. World J Hepatol. 2023;15(12):1272-1283.[DOI]
-
30. Deng D, Yang S, Yu X, Zhou R, Liu Y, Zhang H, et al. Aging‐induced short‐chain acyl‐CoA dehydrogenase promotes age‐related hepatic steatosis by suppressing lipophagy. Aging Cell. 2024;23(10):e14256.[DOI]
-
31. Schütz F, Longo L, Keingeski MB, Filippi-Chiela E, Uribe-Cruz C, Álvares-da-Silva MR. Lipophagy and epigenetic alterations are related to metabolic dysfunction-associated steatotic liver disease progression in an experimental model. World J Hepatol. 2024;16(12):1468-1479.[DOI]
-
32. Chen X, Wang L, Denning KL, Mazur A, Xu Y, Wang K, et al. Hepatocyte-Specific PEX16 Abrogation in Mice Leads to Hepatocyte Proliferation, Alteration of Hepatic Lipid Metabolism, and Resistance to High-Fat Diet (HFD)-Induced Hepatic Steatosis and Obesity. Biomedicines. 2024;12(5):988.[DOI]
-
33. Stilkerich A, Schicht G, Seidemann L, Hänsel R, Friebel A, Hoehme S, et al. Cell homeostasis or cell death—The balancing act between autophagy and apoptosis caused by steatosis-induced endoplasmic reticulum (ER) stress. Cells. 2025;14(6):449.[DOI]
-
34. Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun. 2013;4(1):2300.[DOI]
-
35. Liu R, Cui J, Sun Y, Xu W, Wang Z, Wu M, et al. Autophagy deficiency promotes M1 macrophage polarization to exacerbate acute liver injury via ATG5 repression during aging. Cell Death Discov. 2021;7(1):397.[DOI]
-
36. Galle-Treger L, Helou DG, Quach C, Howard E, Hurrell BP, Muench GRA, et al. Autophagy impairment in liver CD11c+ cells promotes non-alcoholic fatty liver disease through production of IL-23. Nat Commun. 2022;13(1):1440.[DOI]
-
37. Al Sayegh R, Wan J, Caër C, Azoulai M, Gasperment M, Baweja S, et al. Defective autophagy in CD4 T cells drives liver fibrosis via type 3 inflammation. Nat Commun. 2025;16(1):3860.[DOI]
-
38. Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, et al. Liver-Specific Loss of Atg5 Causes Persistent Activation of Nrf2 and Protects Against Acetaminophen-Induced Liver Injury. Toxicol Sci. 2012;127(2):438-450.[DOI]
-
39. Baselli GA, Jamialahmadi O, Pelusi S, Ciociola E, Malvestiti F, Saracino M, et al. Rare ATG7 genetic variants predispose patients to severe fatty liver disease. J Hepatol. 2022;77(3):596-606.[DOI]
-
40. Waye MM. Mutation of autophagy-related gene ATG7 increases the risk of severe disease in patients with non-alcoholic fatty liver disease. Liver Res. 2023;7(4):365-366.[DOI]
-
41. Liu P, Anandhan A, Chen J, Shakya A, Dodson M, Ooi A, et al. Decreased autophagosome biogenesis, reduced NRF2, and enhanced ferroptotic cell death are underlying molecular mechanisms of non-alcoholic fatty liver disease. Redox Biol. 2023;59:102570.[DOI]
-
42. Byun S, Seok S, Kim YC, Zhang Y, Yau P, Iwamori N, et al. Fasting-induced FGF21 signaling activates hepatic autophagy and lipid degradation via JMJD3 histone demethylase. Nat Commun. 2020;11(1):807.[DOI]
-
43. Römermann D, Ansari N, Schultz‐Moreira AR, Michael A, Marhenke S, Hardtke‐Wolenski M, et al. Absence of Atg7 in the liver disturbed hepatic regeneration after liver injury. Liver Int. 2020;40(5):1225-1238.[DOI]
-
44. Zhuang Y, Li Y, Li X, Xie Q, Wu M. Atg7 knockdown augments concanavalin A-induced acute hepatitis through an ROS-mediated p38/MAPK pathway. PLoS ONE. 2016;11(3):e0149754.[DOI]
-
45. Zalma BA, Ibrahim M, Rodriguez-Polanco FC, Bhavsar CT, Rodriguez EM, Cararo-Lopes E, et al. Autophagy-related 7 (ATG7) regulates food intake and liver health during asparaginase exposure. J Biol Chem. 2025;301(2):108171.[DOI]
-
46. Sebti S, Zou Z, Shiloh MU. BECN1F121A mutation increases autophagic flux in aged mice and improves aging phenotypes in an organ-dependent manner. Autophagy. 2022;19(3):957-965.[DOI]
-
47. Lin Y, Li Y, Liang G, Yang X, Yang J, Hu Q, et al. Single‐cell transcriptome analysis of aging mouse liver. FASEB J. 2024;38(4):e23473.[DOI]
-
48. Wan J, Weiss E, Ben Mkaddem S, Mabire M, Choinier PM, Picq O, et al. LC3-associated phagocytosis protects against inflammation and liver fibrosis via immunoreceptor inhibitory signaling. Sci Transl Med. 2020;12(539):eaaw8523.[DOI]
-
49. Ogura M, Kaminishi T, Shima T, Torigata M, Bekku N, Tabata K, et al. Microautophagy regulated by STK38 and GABARAPs is essential to repair lysosomes and prevent aging. EMBO Rep. 2023;24(12):e57300.[DOI]
-
50. Deretic V, Klionsky DJ. An expanding repertoire of E3 ligases in membrane Atg8ylation. Nat Cell Biol. 2024;26(3):307-308.[DOI]
-
51. Fernández ÁF, Sebti S, Wei Y, Zou Z, Shi M, McMillan KL, et al. Disruption of the beclin 1–BCL2 autophagy regulatory complex promotes longevity in mice. Nature. 2018;558(7708):136-140.[DOI]
-
52. Chen CL, Huang FF, Lin HF, Wu CC, Ni YH, Lin YC. Tat-Beclin-1 peptide ameliorates metabolic dysfunction-associated steatotic liver disease by enhancing hepatic autophagy. Int J Mol Sci. 2024;25(22):12372.[DOI]
-
53. Schneider JL, Villarroya J, Diaz‐Carretero A, Patel B, Urbanska AM, Thi MM, et al. Loss of hepatic chaperone‐mediated autophagy accelerates proteostasis failure in aging. Aging Cell. 2015;14(2):249-264.[DOI]
-
54. Khawaja RR, Martín-Segura A, Santiago-Fernández O, Sereda R, Lindenau K, McCabe M, et al. Sex-specific and cell-type-specific changes in chaperone-mediated autophagy across tissues during aging. Nat Aging. 2025;5(4):691-708.[DOI]
-
55. Endicott SJ, Boynton DN, Beckmann LJ, Miller RA. Long-lived mice with reduced growth hormone signaling have a constitutive upregulation of hepatic chaperone-mediated autophagy. Autophagy. 2020;17(3):612-625.[DOI]
-
56. Zhang KK, Zhang P, Kodur A, Erturk I, Burns CM, Kenyon C, et al. LAMP2A, and other chaperone-mediated autophagy related proteins, do not decline with age in genetically heterogeneous UM-HET3 mice. Aging. 2023;15(11):4685-4698.[DOI]
-
57. Di Veroli B, Bentanachs R, Roglans N, Alegret M, Giona L, Profumo E, et al. Sex differences affect the NRF2 signaling pathway in the early phase of liver steatosis: A high-fat-diet-fed rat model supplemented with liquid fructose. Cells. 2024;13(15):1247.[DOI]
-
58. Sánchez-Mendoza LM, González-Reyes JA, Rodríguez-López S, García-Caballero C, Moreno JA, de Cabo R, et al. Adaptations of mitochondrial, autophagy and nutrient sensing pathways in the liver from long-lived mice overexpressing CYB5R3 are sex-dependent and involve inter-organ responses. GeroScience. 2025.[DOI]
-
59. Campisi D, Hawkins NT, Bonjour K, Wollert T. The role of WIPI2, ATG16L1 and ATG12-ATG5 in selective and nonselective autophagy. J Mol Biol. 2025;437(18):169138.[DOI]
-
60. Liu J, Xiao Y, Cao L, Lu S, Zhang S, Yang R, et al. Insights on E1-like enzyme ATG7: Functional regulation and relationships with aging-related diseases. Commun Biol. 2024;7(1):382.[DOI]
-
61. Wang S, Long H, Hou L, Feng B, Ma Z, Wu Y, et al. The mitophagy pathway and its implications in human diseases. Sig Transduct Target Ther. 2023;8(1):304.[DOI]
-
62. Liu S, Wang L, Zhu L, Zhao T, Han P, Yan F, et al. Mechanism and regulation of mitophagy in liver diseases: A review. Front Cell Dev Biol. 2025;13:1614940.[DOI]
-
63. Yamada T, Murata D, Adachi Y, Itoh K, Kameoka S, Igarashi A, et al. Mitochondrial stasis reveals p62-mediated ubiquitination in parkin-independent mitophagy and mitigates nonalcoholic fatty liver disease. Cell Metab. 2018;28(4):588-604.[DOI]
-
64. Tostes K, dos Santos AC, Alves LO, Bechara LRG, Marascalchi R, Macabelli CH, et al. Autophagy deficiency abolishes liver mitochondrial DNA segregation. Autophagy. 2022;18(10):2397-2408.[DOI]
-
65. Li R, Wang Z, Wang Y, Sun R, Zou B, Tian X, et al. SIRT3 regulates mitophagy in liver fibrosis through deacetylation of PINK1/NIPSNAP1. J Cell Physiol. 2023;238(9):2090-2102.[DOI]
-
66. Hu Z, Zhang H, Wang Y, Li B, Liu K, Ran J, et al. Exercise activates Sirt1-mediated Drp1 acetylation and inhibits hepatocyte apoptosis to improve nonalcoholic fatty liver disease. Lipids Health Dis. 2023;22(1):33.[DOI]
-
67. Huang L, Zeng X, Li B, Wang C, Zhou M, Lang H, et al. Dihydromyricetin attenuates palmitic acid-induced oxidative stress by promoting autophagy via SIRT3-ATG4B signaling in hepatocytes. Nutr Metab. 2021;18(1):83.[DOI]
-
68. Zhang Y, Gong C, Tao L, Zhai J, Huang F, Zhang S. Involvement of SIRT1-mediated aging in liver diseases. Front Cell Dev Biol. 2025;13:1548015.[DOI]
-
69. Adjei‐Mosi J, Sun Q, Smithson SB, Shealy GL, Amerineni KD, Liang Z, et al. Age‐dependent loss of hepatic SIRT1 enhances NLRP3 inflammasome signaling and impairs capacity for liver fibrosis resolution. Aging Cell. 2023;22(5):e13811.[DOI]
-
70. Ruart M, Chavarria L, Campreciós G, Suárez-Herrera N, Montironi C, Guixé-Muntet S, et al. Corrigendum to: “Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury” [J Hepatol (2019) 458-469]. J Hepatol. 2020;73(3):744.[DOI]
-
71. Ortega-Molina A, Lebrero-Fernández C, Sanz A, Calvo-Rubio M, Deleyto-Seldas N, de Prado-Rivas L, et al. A mild increase in nutrient signaling to mTORC1 in mice leads to parenchymal damage, myeloid inflammation and shortened lifespan. Nat Aging. 2024;4(8):1102-1120.[DOI]
-
72. Plata-Gómez AB, de Prado-Rivas L, Sanz A, Deleyto-Seldas N, García F, de la Calle Arregui C, et al. Hepatic nutrient and hormone signaling to mTORC1 instructs the postnatal metabolic zonation of the liver. Nat Commun. 2024;15(1):1878.[DOI]
-
73. Xu F, Tautenhahn HM, Dirsch O, Dahmen U. Modulation of autophagy: a novel “rejuvenation” strategy for the aging liver. Oxid Med Cell Longev. 2021;2021(1):6611126.[DOI]
-
74. Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Sig Transduct Target Ther. 2022;7(1):391.[DOI]
-
75. Chao X, Wang S, Fulte S, Ma X, Ahamed F, Cui W, et al. Hepatocytic p62 suppresses ductular reaction and tumorigenesis in mouse livers with mTORC1 activation and defective autophagy. J Hepatol. 2022;76(3):639-651.[DOI]
-
76. Menon S, Yecies JL, Zhang HH, Howell JJ, Nicholatos J, Harputlugil E, et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal. 2012;5(217):ra24.[DOI]
-
77. Jang Y, Ko M, Lee JY, Kim JY, Lee EW, Kwon HJ. Inhibition of lysosomal LAMTOR1 increases autophagy by suppressing the MTORC1 pathway to ameliorate lipid accumulations in MAFLD. Autophagy. 2025.[DOI]
-
78. Ding H, Ge G, Tseng Y, Ma Y, Zhang J, Liu J. Hepatic autophagy fluctuates during the development of non-alcoholic fatty liver disease. Ann Hepatol. 2020;19(5):516-522.[DOI]
-
79. Sun H, Ni HM, McCracken JM, Akakpo JY, Fulte S, McKeen T, et al. Liver-specific deletion of mechanistic target of rapamycin does not protect against acetaminophen-induced liver injury in mice. Liver Res. 2021;5(2):79-87.[DOI]
-
80. Xu M, Wang H, Wang J, Burhan D, Shang R, Wang P, et al. mTORC2 signaling is necessary for timely liver regeneration after partial hepatectomy. Am J Pathol. 2020;190(4):817-829.[DOI]
-
81. Wang ZY, Chen RX, Wang JF, Liu SC, Xu X, Zhou T, et al. Apolipoprotein A-1 accelerated liver regeneration through regulating autophagy via AMPK-ULK1 pathway. Cell Mol Gastroenterol Hepatol. 2024;17(4):539-551.[DOI]
-
82. Li Q, Xiao N, Zhang H, Liang G, Lin Y, Qian Z, et al. Systemic aging and aging‐related diseases. FASEB J. 2025;39(5):e70430.[DOI]
-
83. Desjardins EM, Smith BK, Day EA, Ducommun S, Sanders MJ, Nederveen JP, et al. The phosphorylation of AMPKβ1 is critical for increasing autophagy and maintaining mitochondrial homeostasis in response to fatty acids. Proc Natl Acad Sci U.S.A. 2022;119(48):e2119824119.[DOI]
-
84. Qu Q, Chen Y, Wang Y, Wang W, Long S, Yang HY, et al. Lithocholic acid binds TULP3 to activate sirtuins and AMPK to slow down ageing. Nature. 2024;643(8070):201-209.[DOI]
-
85. Park JM, Lee DH, Kim DH. Redefining the role of AMPK in autophagy and the energy stress response. Nat Commun. 2023 May 24;14(1):2994.[DOI]
-
86. Longo M, Bishnu A, Risiglione P, Montava-Garriga L, Cuenco J, Sakamoto K, et al. Opposing roles for AMPK in regulating distinct mitophagy pathways. Mol Cell. 2024;84(22):4350-4367.[DOI]
-
87. Tian C, Huang R, Xiang M. SIRT1: Harnessing multiple pathways to hinder NAFLD. Pharmacol Res. 2024;203:107155.[DOI]
-
88. Han C, Gu Y, Shan H, Mi W, Sun J, Shi M, et al. O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat Commun. 2017;8(1):1491.[DOI]
-
89. Peyman M, Babin-Ebell A, Rodríguez-Rodríguez R, Rigon M, Aguilar-Recarte D, Villarroya J, et al. SIRT1 regulates hepatic vldlr levels. Cell Commun Signal. 2024;22(1):297.[DOI]
-
90. Varghese B, Chianese U, Capasso L, Sian V, Bontempo P, Conte M, et al. SIRT1 activation promotes energy homeostasis and reprograms liver cancer metabolism. J Transl Med. 2023;21(1):627.[DOI]
-
91. Feng L, Chen M, Li Y, Li M, Hu S, Zhou B, et al. Sirt1 deacetylates and stabilizes p62 to promote hepato-carcinogenesis. Cell Death Dis. 2021;12(4):405.[DOI]
-
92. Fu X, Qie J, Chen J, Gao Z, Li X, Feng S, et al. Inhibition of SIRT1 relieves hepatocarcinogenesis via alleviating autophagy and inflammation. Int J Biol Macromol. 2024;278:134120.[DOI]
-
93. Chan HY, Ramasamy TS, Chung FFL, Teow SY. Role of sirtuin 1 (SIRT1) in regulation of autophagy and nuclear factor-kappa Beta (NF-ĸβ) pathways in sorafenib-resistant hepatocellular carcinoma (HCC). Cell Biochem Biophys. 2024;82(2):959-968.[DOI]
-
94. Dinić M, Lukić J, Djokić J, Milenković M, Strahinić I, Golić N, et al. Lactobacillus fermentum Postbiotic-induced Autophagy as Potential Approach for Treatment of Acetaminophen Hepatotoxicity. Front Microbiol. 2017;8:594.[DOI]
-
95. Jin D, Jia C, Yang B, Wu Y, Chen L, Liu R, et al. The ameliorative mechanism of Lactiplantibacillus plantarum NJAU-01 against d-galactose induced oxidative stress: a hepatic proteomics and gut microbiota analysis. Food Funct. 2024;15(11):6174-6188.[DOI]
-
96. Yin Y, Xie Y, Wu Z, Qian Q, Yang H, Li S, et al. Preventive effects of apple polyphenol extract on high-fat-diet-induced hepatic steatosis are related to the regulation of hepatic lipid metabolism, autophagy, and gut microbiota in aged Mice. J Agric Food Chem. 2023;71(50):20011-20033.[DOI]
-
97. Yan S, Khambu B, Chen X, Dong Z, Guo G, Yin XM. Hepatic autophagy deficiency remodels gut microbiota for adaptive protection via FGF15-FGFR4 signaling. Cell Mol Gastroenterol Hepatol. 2021;11(4):973-997.[DOI]
-
98. Grajeda-Iglesias C, Durand S, Daillère R, Iribarren K, Lemaitre F, Derosa L, et al. Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging. 2021;13(5):6375-6405.[DOI]
-
99. Tanaka S, Hikita H, Tatsumi T, Sakamori R, Nozaki Y, Sakane S, et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology. 2016;64(6):1994-2014.[DOI]
-
100. Lan T, Li Q, Yu M, Duan X, Ming T, Li S, et al. Dual-targeted siRubicon delivery strategy triggers hepatocellular lipophagy for mitigating liver steatosis. Nat Commun. 2025;16(1):7455.[DOI]
-
101. Bhargava HK, Tabata K, Byck JM, Hamasaki M, Farrell DP, Anishchenko I, et al. Structural basis for autophagy inhibition by the human Rubicon–Rab7 complex. Proc Natl Acad Sci U.S.A. 2020;117(29):17003-17010.[DOI]
-
102. Li F, Zhang H. Lysosomal acid lipase in lipid metabolism and beyond. Arterioscler Thromb Vasc Biol. 2019;39(5):850-856.[DOI]
-
103. Minami Y, Hoshino A, Higuchi Y, Hamaguchi M, Kaneko Y, Kirita Y, et al. Liver lipophagy ameliorates nonalcoholic steatohepatitis through extracellular lipid secretion. Nat Commun. 2023;14(1):4084.[DOI]
-
104. Peng Z, Gong Y, Wang X, He W, Wu L, Zhang L, et al. METTL3-m6A-Rubicon axis inhibits autophagy in nonalcoholic fatty liver disease. Mol Ther. 2022;30(2):932-946.[DOI]
-
105. Shiode Y, Hikita H, Tanaka S, Shirai K, Doi A, Sakane S, et al. Hepatitis C virus enhances Rubicon expression, leading to autophagy inhibition and intracellular innate immune activation. Sci Rep. 2020;10(1):15290.[DOI]
-
106. Dong F, Hu XW, Zhang S, He F, Naz A, He L, et al. Rubicon deficiency exacerbates fasting-induced hepatic steatosis. J Bio-X Res. 2022;5(01):35-41.[DOI]
-
107. Yamamuro T, Kawabata T, Fukuhara A, Saita S, Nakamura S, Takeshita H, et al. Age-dependent loss of adipose Rubicon promotes metabolic disorders via excess autophagy. Nat Commun. 2020;11(1):4150.[DOI]
-
108. Pei Q, Yi Q, Tang L. Liver fibrosis resolution: from molecular mechanisms to therapeutic opportunities. Int J Mol Sci. 2023;24(11):9671.[DOI]
-
109. Hernández-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142(4):938-946.[DOI]
-
110. Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, et al. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020;73(5):1144-1154.[DOI]
-
111. Tao Y, Qiu T, Yao X, Jiang L, Wang N, Jia X, et al. Autophagic-CTSB-inflammasome axis modulates hepatic stellate cells activation in arsenic-induced liver fibrosis. Chemosphere. 2020;242:124959.[DOI]
-
112. Jiang N, Zhang J, Ping J, Xu L. Salvianolic acid B inhibits autophagy and activation of hepatic stellate cells induced by TGF-β1 by downregulating the MAPK pathway. Front Pharmacol. 2022;13:938856.[DOI]
-
113. Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14(7):397-411.[DOI]
-
114. Yuan Y, Li J, Chen M, Zhao Y, Zhang B, Chen X, et al. Nano-encapsulation of drugs to target hepatic stellate cells: Toward precision treatments of liver fibrosis. J Control Release. 2024;376:318-336.[DOI]
-
115. Dudek M, Swift J, Meng QJ. The circadian clock and extracellular matrix homeostasis in aging and age-related diseases. Am J Physiol Cell Physiol. 2023;325(1):C52-C59.[DOI]
-
116. Guo HY, Yu XN, Zhang GC, Yin J, Dong L, Liu TT, et al. Increased expression of autophagy‐related gene 5 indicates poor prognosis in patients with hepatocellular carcinoma. J Dig Dis. 2023;24(6-7):399-407.[DOI]
-
117. Shayeb AE, Deghedy A, Bedewy ES, Badawy S, Abdeen N. Serum Beclin 1 and autophagy-related protein-5 and the risk of hepatocellular carcinoma among cirrhotic hepatitis C patients. Egypt Liver J. 2021;11(1):81.[DOI]
-
118. Barthet VJA, Brucoli M, Ladds MJGW, Nössing C, Kiourtis C, Baudot AD, et al. Autophagy suppresses the formation of hepatocyte-derived cancer-initiating ductular progenitor cells in the liver. Sci Adv. 2021;7(23):eabf9141.[DOI]
-
119. Hashemi M, Nadafzadeh N, Imani MH, Rajabi R, Ziaolhagh S, Bayanzadeh SD, et al. Targeting and regulation of autophagy in hepatocellular carcinoma: revisiting the molecular interactions and mechanisms for new therapy approaches. Cell Commun Signal. 2023;21(1):32.[DOI]
-
120. Deust A, Chobert MN, Demontant V, Gricourt G, Denaës T, Thiolat A, et al. Macrophage autophagy protects against hepatocellular carcinogenesis in mice. Sci Rep. 2021;11(1):18809.[DOI]
-
121. Ferrín G, Guerrero M, Amado V, Rodríguez-Perálvarez M, De la Mata M. Activation of mTOR signaling pathway in hepatocellular carcinoma. Int J Mol Sci. 2020;21(4):1266.[DOI]
-
122. Rahdan F, Abedi F, Dianat-Moghadam H, Sani MZ, Taghizadeh M, Alizadeh E. Autophagy-based therapy for hepatocellular carcinoma: from standard treatments to combination therapy, oncolytic virotherapy, and targeted nanomedicines. Clin Exp Med. 2024;25(1):13.[DOI]
-
123. Zhang Y, Wang H, Xu C, Ye X, Nan Y, Hu X, et al. Rubicon siRNA-encapsulated liver-targeting nanoliposome is a promising therapeutic for non-alcoholic fatty liver disease. Int J Pharm. 2025;672:125291.[DOI]
-
124. Yao Y, Civelek AC, Li XF. The application of 18F-FDG PET/CT imaging for human hepatocellular carcinoma: A narrative review. Quant Imaging Med Surg. 2023;13(9):6268.[DOI]
-
125. Mizushima N, Murphy LO. Autophagy assays for biological discovery and therapeutic development. Trends Biochem Sci. 2020;45(12):1080-1093.[DOI]
-
126. Izquierdo M, de Souto Barreto P, Arai H, Bischoff-Ferrari HA, Cadore EL, Cesari M, et al. Global consensus on optimal exercise recommendations for enhancing healthy longevity in older adults (ICFSR). J Nutr Health Aging. 2025;29(1):100401.[DOI]
-
127. Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328(5976):321-326.[DOI]
-
128. Di Daniele N, Marrone G, Di Lauro M, Di Daniele F, Palazzetti D, Guerriero C, et al. Effects of caloric restriction diet on arterial hypertension and endothelial dysfunction. Nutrients. 2021;13(1):274.[DOI]
-
129. Lopes S, Afreixo V, Teixeira M, Garcia C, Leitão C, Gouveia M, et al. Exercise training reduces arterial stiffness in adults with hypertension: a systematic review and meta-analysis. J Hypertens. 2020;39(2):214-222.[DOI]
-
130. Castro-Barquero S, Ruiz-León AM, Sierra-Pérez M, Estruch R, Casas R. Dietary strategies for metabolic syndrome: A comprehensive review. Nutrients. 2020;12(10):2983.[DOI]
-
131. Liang M, Pan Y, Zhong T, Zeng Y, Cheng AS. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: a systematic review and network meta-analysis. Rev Cardiovasc Med. 2021;22(4):1523-1533.[DOI]
-
132. Zhu W. Effective roles of exercise and diet adherence in non-alcoholic fatty liver disease. World J Gastroenterol. 2024;30(29):3456-3460.[DOI]
-
133. Angulo J, El Assar M, Álvarez-Bustos A, Rodríguez-Mañas L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020;35:101513.[DOI]
-
134. Castro-Barquero S, Ruiz-León AM, Sierra-Pérez M, Estruch R, Casas R. Dietary strategies for metabolic syndrome: a comprehensive review. Nutrients. 2020;12(10):2983.[DOI]
-
135. Chung KW, Chung HY. The effects of calorie restriction on autophagy: Role on aging intervention. Nutrients. 2019;11(12):2923.[DOI]
-
136. Normand-Gravier T, Solsona R, Arnould F, Deriaz R, Bertrand-Gaday C, Borrani F, et al. Acute effects of heat intervention and hybrid exercise on protein synthesis, ribosome biogenesis and autophagy. J Therm Biol. 2025;131:104169.[DOI]
-
137. Martinez-Canton M, Galvan-Alvarez V, Gallego-Selles A, Gelabert-Rebato M, Garcia-Gonzalez E, Gonzalez-Henriquez JJ, et al. Activation of macroautophagy and chaperone-mediated autophagy in human skeletal muscle by high-intensity exercise in normoxia and hypoxia and after recovery with or without post-exercise ischemia. Free Radic Biol Med. 2024;222:607-624.[DOI]
-
138. Kepp O, Chen G, Carmona-Gutierrez D, Madeo F, Kroemer G. A discovery platform for the identification of caloric restriction mimetics with broad health-improving effects. Autophagy. 2020;16(1):188-189.[DOI]
-
139. Shi B, Wang W, Ye M, Liang M, Yu Z, Zhang Y, et al. Spermidine suppresses the activation of hepatic stellate cells to cure liver fibrosis through autophagy activator MAP1S. Liver Int. 2023;43(6):1307-1319.[DOI]
-
140. Ni Y, Hu Y, Lou X, Rong N, Liu F, Yang C, et al. Spermidine ameliorates nonalcoholic steatohepatitis through thyroid hormone-responsive protein signaling and the gut microbiota-mediated metabolism of bile acids. J Agric Food Chem. 2022;70(21):6478-6492.[DOI]
-
141. Gao M, Zhao W, Li C, Xie X, Li M, Bi Y, et al. Spermidine ameliorates non-alcoholic fatty liver disease through regulating lipid metabolism via AMPK. Biochem Biophys Res Commun. 2018;505(1):93-98.[DOI]
-
142. Singh AK, Garg G, Singh S, Rizvi SI. Synergistic effect of rapamycin and metformin against age-dependent oxidative stress in rat erythrocytes. Rejuvenation Res. 2017;20(5):420-429.[DOI]
-
143. Du X, Han X, Zhu J, Zhang Z, Jin H, Zhang D, et al. Rapamycin ameliorates intrahepatic inflammation in MASLD by increasing macrophage fatty acid oxidation levels. Int Immunopharmacol. 2025;163:115288.[DOI]
-
144. Holzhütter HG, Hudert CA, Berndt N. Patient-specific effects of metformin on the hepatic metabolism in adolescents with metabolic dysfunction-associated steatotic liver disease (MASLD). J Mol Med. 2025;103:837-847.[DOI]
-
145. Carpi GC, Campos LR, Kopacek C. Metformin seems promising in improving liver enzymes in pediatric MASLD. Eur J Pediatr. 2025;184(7):443.[DOI]
-
146. Palomer X, Wang JR, Escalona C, Wu S, Wahli W, Vázquez-Carrera M. Targeting AMPK as a potential treatment for hepatic fibrosis in MASLD. Trends Pharmacol Sci. 2025;46(6):551-566.[DOI]
-
147. Wan T, Chen Z, Li J, Yuan X, Zheng M, Qin L, et al. AMPK agonist AICAR ameliorates maternal hepatic lipid metabolism disorder, inflammation, and fibrosis caused by PM2.5 exposure during pregnancy. Sci Rep. 2025;15(1):8689.[DOI]
-
148. Montégut L, Lambertucci F, Moledo-Nodar L, Fiuza-Luces C, Rodríguez-López C, Serra-Rexach JA, et al. Acyl-CoA-binding protein as a driver of pathological aging. Proc Natl Acad Sci U.S.A. 2025;122(28):e2501584122.[DOI]
-
149. Li S, Motiño O, Lambertucci F, Pol J, Chen H, Pan L, et al. Neutralization of acyl coenzyme A binding protein for the experimental prevention and treatment of hepatocellular carcinoma. Cell Rep Med. 2025;6(7):102232.[DOI]
-
150. Motiño O, Lambertucci F, Anagnostopoulos G, Li S, Nah J, Castoldi F, et al. ACBP/DBI protein neutralization confers autophagy-dependent organ protection through inhibition of cell loss, inflammation, and fibrosis. Proc Natl Acad Sci U.S.A. 2022;119(41):e2207344119.[DOI]
-
151. Motiño O, Lambertucci F, Joseph A, Durand S, Anagnostopoulos G, Li S, et al. ACBP/DBI neutralization for the experimental treatment of fatty liver disease. Cell Death Differ. 2025;32(3):434-446.[DOI]
-
152. Jalali P, Shahmoradi A, Samii A, Mazloomnejad R, Hatamnejad MR, Saeed A, et al. The role of autophagy in cancer: From molecular mechanism to therapeutic window. Front Immunol. 2025;16:1528230.[DOI]
-
153. Tabibzadeh S. Role of autophagy in aging: The good, the bad, and the ugly. Aging cell. 2023;22(1):e13753.[DOI]
-
154. Mundo Rivera VM, Tlacuahuac Juárez JR, Murillo Melo NM, Leyva Garcia N, Magaña JJ, Cordero Martínez J, et al. Natural autophagy activators to fight age-related diseases. Cells. 2024;13(19):1611.[DOI]
-
155. Ames ML, Karlsen MC, Sundermeir SM, Durrwachter N, Hemmingson TA, Reznar MM, et al. Lifestyle medicine implementation in 8 health systems: Protocol for a multiple case study investigation. JMIR Res Protoc. 2024;13(1):e51562.[DOI]
-
156. Madeo F, Hofer SJ, Pendl T, Bauer MA, Eisenberg T, Carmona-Gutierrez D, et al. Nutritional aspects of spermidine. Annu Rev Nutr. 2020;40:135-159.[DOI]
-
157. Hofer SJ, Daskalaki I, Bergmann M, Friščić J, Zimmermann A, Mueller MI, et al. Spermidine is essential for fasting-mediated autophagy and longevity. Nat Cell Biol. 2024;26(9):1571-1584.[DOI]
-
158. Zou D, Zhao Z, Li L, Min Y, Zhang D, Ji A, et al. A comprehensive review of spermidine: Safety, health effects, absorption and metabolism, food materials evaluation, physical and chemical processing, and bioprocessing. Compr Rev Food Sci Food Saf. 2022;21(3):2820-2842.[DOI]
-
159. Nguyen LS, Vautier M, Allenbach Y, Zahr N, Benveniste O, Funck-Brentano C, et al. Sirolimus and mTOR inhibitors: a review of side effects and specific management in solid organ transplantation. Drug Saf. 2019;42(7):813-825.[DOI]
-
160. Feng J, Wang X, Ye X, Ares I, Lopez-Torres B, Martínez M, et al. Mitochondria as an important target of metformin: The mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res. 2022;177:106114.[DOI]
-
161. D’Errico S, Oliviero G, Borbone N, Amato J, Piccialli V, Varra M, et al. Synthesis of new acadesine (AICA-riboside) analogues having acyclic D-ribityl or 4-hydroxybutyl chains in place of the ribose. Molecules. 2013;18(8):9420-9431.[DOI]
-
162. Mizushima N. Autophagic flux measurement: Cargo degradation versus generation of degradation products. Curr Opin Cell Biol. 2025;93:102463.[DOI]
-
163. Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th Edition)1. Autophagy. 2021;17(1):1-382.[DOI]
-
164. Minami S, Nakamura S, Yoshimori T. Rubicon in metabolic diseases and ageing. Front Cell Dev Biol. 2022;9:816829.[DOI]
-
165. Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009-1017.[DOI]
-
166. Sun X, Li F, Yan H, Chang X, Yao X, Yang X, et al. Intermittent compared with continuous calorie restriction for treatment of metabolic dysfunction-associated steatotic liver disease: A randomized clinical trial. Am J Clin Nutr. 2025;121(1):158-166.[DOI]
-
167. Lee HA, Moon H, Kim Y, Lee HA, Kim HY. Effect of 12-week intermittent calorie restriction compared to standard of care in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Trials. 2023;24(1):490.[DOI]
-
168. Bagnato CB, Bianco A, Bonfiglio C, Franco I, Verrelli N, Carella N, et al. Healthy lifestyle changes improve cortisol levels and liver steatosis in MASLD patients: results from a randomized clinical trial. Nutrients. 2024;16(23):4225.[DOI]
-
169. Shojaee-Moradie F, Cuthbertson DJ, Barrett M, Jackson NC, Herring R, Thomas EL, et al. Exercise training reduces liver fat and increases rates of VLDL clearance but not VLDL production in NAFLD. J Clin Endocrinol Metab. 2016;101(11):4219-4228.[DOI]
-
170. Liu L, Wang Q, Li Y, Cai J, Wang Y, Li Y, et al. TAT‐beclin1 treatment accelerates the development of atherosclerotic lesions in ApoE‐deficient mice. FASEB J. 2024;38(13):e23765.[DOI]
-
171. Xiao W, Chen M, Peng Q, Sha K, Liu T, Xia J, et al. Lithocholic acid promotes rosacea-like skin inflammation via G protein-coupled bile acid receptor 1. Biochim Biophys Acta Mol Basis Dis. 2022;1868(12):166563.[DOI]
-
172. Duboc H, Aelion H, Rainteau D, Rajca S, Sokol H, Humbert L, et al. Crosstalk between the hepatologist and the cardiologist: a future place for the lithocholic acid as a coronary atheroma risk factor? Hepatology. 20121;56(6):2426.[DOI]
-
173. Mangano DT. Effects of acadesine on myocardial infarction, stroke, and death following surgery: a meta-analysis of the 5 international randomized trials. JAMA. 1997;277(4):325-332.[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite