Kroemer G, Centre de Recherche des Cordeliers, Equipe labellisée par la Ligue contre le cancer, Université Paris Cité, Sorbonne Université, Inserm U1138, Institut Universitaire de France, Paris 75006, France. E-mail: kroemer@orange.fr
Abstract
The biology of aging is increasingly understood through geroscience frameworks integrating molecular, cellular, physiological, and social hallmarks. Recently, we introduced psychosocial factors including mental illness as an important hallmark of aging. Indeed, exposome-centered approaches reveal complex interactions among socioeconomic, environmental, behavioral, and genomic factors. Precision Geromedicine aims to target all these determinants in a holistic fashion to improve aging trajectories and extend healthspan.
Keywords
1. Introduction
Over recent years, there has been a large and growing interest in the study of aging due to its multiple scientific, medical, and socioeconomic implications. In fact, the proportion of older individuals is increasing worldwide, and demographic studies have concluded that by 2030, people older than 60 will represent one-sixth of the planet’s population, rising further to one-fifth by 2050[1]. Emerging areas of knowledge, such as Geroscience and Geromedicine, have already started to guide progress toward an improved understanding of the molecular mechanisms of normal and pathological aging, including age-related diseases such as cancer, metabolic disorders, neurodegenerative processes, cardiovascular diseases, and musculoskeletal alterations[2,3]. The final goal of such geroscientific and geromedical approaches is to optimize health and postpone, prevent, or treat age-related diseases across the lifespan. In this context, the introduction of the so-called ‘hallmarks of aging’ has provided an integrative framework to conceptualize and organize the extremely complex field of aging research[4,5] (Figure 1).
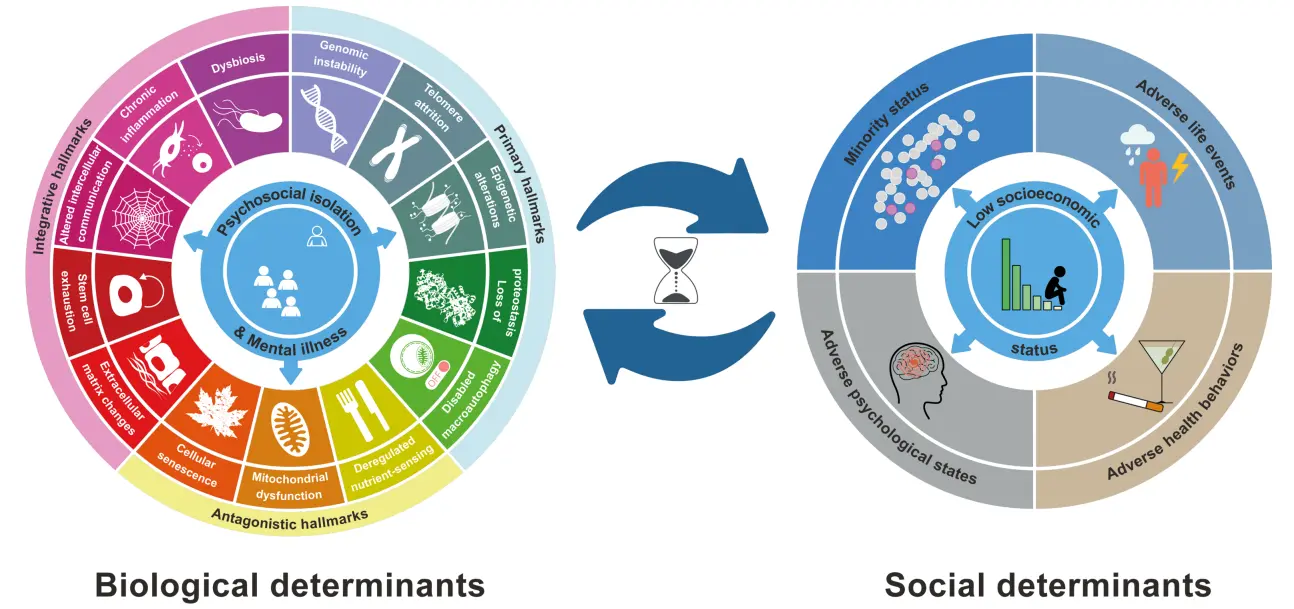
Figure 1. Biological and social determinants of aging. Data for the 14 biological hallmarks of aging have been modifed from Kroemer et al.[3], while the information for the five social hallmarks of aging is based on E.M. Crimmins[13]. Notably, we have modified the previous definition of the 14th hallmark of biological aging from Psychosocial isolation to Psychosocial isolation and mental illness to acknowledge the impact of psychiatric factors on the acceleration of the aging process.
We first defined the hallmarks of aging as molecular or cellular processes that must fulfill three stringent criteria: (i) manifestation during physiological aging, (ii) acceleration of aging when experimentally accentuated, and (iii) deceleration of aging upon experimental attenuation. On this basis, we initially enumerated nine determinants of aging, that were subsequently extended to twelve hallmarks, namely: “genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, disabled autophagy, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis”[5]. These molecular and cellular determinants of aging were categorized into three ensembles: ‘primary’ hallmarks that act as drivers of the aging process; ‘antagonist’ hallmarks that represent protective responses to the primary alterations but become detrimental when they become chronic or their intensity is exacerbated; and ‘integrative’ hallmarks that ultimately account for age-associated functional decline[4].
Very recently, as part of our proposal for advancing from Geroscience to Precision Geromedicine, we have added two new members to the census of hallmarks of aging, namely, (i) extracellular matrix (ECM) changes and (ii) psychosocial isolation[3].
The inclusion of ECM alterations as part of the integrative group of molecular and cellular determinants of aging resulted from the growing body of clinical and experimental evidence that a gradual and bodywide reduction of ECM viscoelasticity accompanies the aging process[6]. Moreover, ECM deficiencies have a strong impact on aging determinants such as mitochondrial homeostasis, tissue fibrosis, cellular senescence and stem cell exhaustion[7-9]. Furthermore, the transfer of an ECM gene encoding hyaluronic acid synthase 2 from long-lived naked mole rats into the genome of mice was shown to reduce cancer incidence, improve musculoskeletal functions, and extend healthspan and lifespan of these transgenic animals[10].
The introduction of adaptation to stress as one of the pillars of aging[2,11], and the definition of ‘psychosocial isolation’ as the 14th hallmark of aging[3] (Figure 1), represent important steps toward the bona fide consideration of social and behavioral factors as essential determinants of organismal aging. Of note, for the sake of the present discussion, we prefer to extend the denomination of this 14th hallmark of aging[3] to ‘psychosocial isolation and mental disease’. This psychosocial and psychiatric aspect has been often neglected, as many efforts in this field have focused predominantly on molecular mechanisms and signaling pathways underlying this extremely complex and dynamic biological process. As part of our work aimed at analyzing aging factors in an integrative manner, this article deals with the social hallmarks of aging and their relationship with the biological determinants associated with the development and progression of aging and age-related diseases.
2. Interactions between Social and Molecular Hallmarks of Aging
The field of sociobiology typically recognizes three major social hallmarks of health: socioeconomic status, social integration, and early life adversities or adverse childhood experiences[11,12]. Over recent years, the growing field of geroscience has tried to integrate these social determinants of health into the context of molecular and cellular mechanisms underlying aging. Previous studies had already shown that a significant part of the observed variability in human aging is influenced by these social factors, but Eileen Crimmins’ pioneering work aimed to integrate the social hallmarks of aging with molecular parameters derived from the geroscientific hallmarks of aging, as well as from the signaling pathways mediating their downstream effects[13]. This approach provided valuable insights into the relative contribution of different social and biological factors to age-related health outcomes[13].
Comparative analysis of both sets of aging hallmarks showed that, following the same criteria used to define the biological determinants of aging, increased levels of social adversity are linked with accelerated aging, whereas reduced exposure to any of the enumerated social factors delays or mitigates the onset of unfavorable health outcomes during aging. Likewise, and also illustrating their close parallels with somatic hallmarks of aging, the social determinants exhibit extensive interconnectedness rather than independence. Interestingly, and consistent with social theories about the fundamental causes of poor health in many modern societies, Crimmins suggested that low socioeconomic status may be the most important social hallmark of aging due to its detrimental impact across all other social determinants of health during the life course[13] (Figure 1). This stands in marked contrast to the apparent absence of hierarchical organization among the otherwise interconnected molecular and cellular hallmarks of aging, as all of them possess comparable potential to contribute to the development of age-related phenotypes[4,5].
Another interesting link between the two sets of aging determinants is the early-life influence of both social and biological hallmarks on aging and disease. Several studies have provided evidence that social problems experienced at early stages in life may affect the individual aging rhythms and trajectories, ultimately precipitating the onset of age-related chronic disorders[14]. In this regard, there is strong evidence from human epidemiological studies and clinical trials, as well as from in vivo experiments with animal models, indicating that social factors acting from infancy and childhood are critical determinants of organismal health and longevity. Poverty, social stress, and psychosocial isolation increase the probability of developing many somatic and mental diseases, enhance the severity of symptoms after viral infections, exacerbate inflammatory reactions, and correlate with accelerated aging, as well as with increased morbidity and mortality rates[12,15,16].
Moreover, old age is frequently accompanied by physical and cognitive decline, leading to progressive social withdrawal, which contributes to psychological or psychiatric alterations, including disruption of biological rhythms, anxiety, depression, and loneliness[17]. These conditions are intrinsically pathogenic, partly due to the chronic activation of neuroendocrine stress reactions, ultimately compromising health and accelerating aging[18]. Indeed, social support is routinely quantified in clinical assessments, and lack of social support is regarded as a prognostic indicator of poor health[19].
Reciprocally, the presence of adequate social relationships positively regulates human physiology from early life, as demonstrated in studies on maternal separation and maternal immune activation[20]. Likewise, epidemiological studies have reported that individuals with high social integration exhibit lower morbidity and mortality[21]. Clinical trials also show that psychosocial group rehabilitation not only enhances subjective health but also reduces mortality in older adults[22]. Similarly, multigenerational communities offer optimal social support for older adults, significantly improving health outcomes[23]. Consistent with these observations, social interaction between adult and old mice increases healthy longevity of the latter[24]. Phylogenetic comparative analyses of approximately 1,000 mammalian species further indicate that solitary species generally live shorter than group-living species[25]. Brain transcriptomic studies have identified genes and immunity-related pathways linking social organization with longevity, providing molecular support for the evolutionary correlation between social factors and aging dynamics[25].
Notably, epidemiological evidence indicates that differences in socioeconomic status, along with associated factors such as absence of social support and loneliness, can shift the onset of certain age-related diseases by more than a decade, adversely affecting healthspan and lifespan[12]. Recently, Legaz et al. demonstrated that structural inequality, defined as the uneven distribution of resources and opportunities, strongly impacts aging and health outcomes, as assessed by a study enrolling a total of 2,135 healthy controls and patients with neurodegenerative diseases from Latin America and the United States[26]. Kivimaki et al. provided further support by showing that life-course social disadvantage accelerates biological aging[27]. This large multicohort study analyzed proteomic signatures linking social disadvantage with specific age-related pathologies, revealing that affected individuals had increased risk for 66 age-related diseases and that, statistically, 39% of these associations were mediated by shifts in the plasma concentrations of 14 age-related proteins. Further analysis identified the pro-inflammatory NF-κB pathway and its downstream effector interleukin-8 as central biological mechanisms linking social disadvantage to aging acceleration[27].
Multiple factors contribute to these adverse health outcomes, including childhood trauma, poor nutrition, sleep disturbances, physical inactivity, chronic exposure to toxins, social discrimination, and psychosocial isolation. Conversely, individuals with somatic or mental health deficits are prone to lower socioeconomic status through the ‘poverty trap’ mechanism, characterized by diminished social motivation, impaired cognition, and reduced economic opportunities[15].
Collectively, these findings support the bidirectional interdependence between social and biological hallmarks in determining health and longevity[3,13,28,29] (Figure 1). Future collaborative research between social scientists and molecular biologists will be essential for elucidating the precise mechanisms through which social circumstances intersect with biological pathways, ultimately shaping individual aging trajectories and the development of age-associated pathologies.
3. Towards the Integration of Biological and Social Hallmarks of Aging
During the life course, social and behavioral factors interact in complex, dynamic networks with biological pathways, collectively shaping health trajectories and aging outcomes. Recent advances in defining the social and biological hallmarks of aging constitute a critical step toward a holistic, integrative understanding of these processes[30] (Figure 1).
As noted by Crimmins[13], the introduction of the notion of ‘allostatic load’ to quantify accumulated chronic stress represented a pioneering effort to incorporate multisystem biological alterations, including those modulated by social determinants, into the study of health, well-being, and aging. Allostasis represents a cumulative metric of physiological dysregulation, and numerous studies have validated its pivotal role in linking socioeconomic status with poor health outcomes[31,32]. Chronic activation of allostatic responses results in an exacerbated allostatic load state that leads to functional decline, induces premature and accelerated aging, and finally reduces longevity and increases mortality in human[31,32]. Recent studies in the allostasis field have further extended the idea that adverse socioeconomic conditions, such as deficient housing, neighborhood conflicts and lack of familial support, induce allostatic load and have a strong impact not only on the development of specific chronic diseases, but also on the incidence of multimorbidity among older individuals[33]. There have also been recent advances in the elucidation of molecular links between increased allostatic load linked with chronic stress and features of accelerated aging. Specifically, cellular allostatic load is associated with significantly enhanced energy expenditure and precocious changes in a series of aging biomarkers such as accelerated telomere shortening, mitochondrial DNA instability, and variations in the DNA-methylation clock[34].
Beyond allostatic load research, recent technological advances across psychosocial and biomedical domains have enabled novel strategies for integrating social and biological determinants of health and longevity. For instance, genomic analyses in a cohort exceeding 350,000 individuals have provided valuable information about genomic variants that—pending confirmation in other cohorts—may be associated with certain aspects of socioeconomic status, as assessed through social markers such as household income, educational attainment, occupational status and signs of social deprivation[35]. These studies identified 56 significant associations involving rare coding variants and uncovered seven novel socioeconomic status –associated genes (CCDC36, EP400, LINC02881, NCAM1, NRN1, RHOB, and TPTEP2-CSNK1E). Additionally, 34 single nucleotide polymorphisms were associated with years of education, and nine with household income. Integration of these genetic factors with health-related phenotypes revealed pleiotropic effects encompassing metabolic disorders and cognitive function, underscoring the genomic contribution to social determinants and their downstream influence on healthspan and lifespan.
Another recent endeavor aimed at integration of social behaviors with their neurobiological substrates involved the development of high-resolution 3D tracking of social interactions and postural dynamics in animal models, including seven rat lines affected by monogenic mutations[36]. This large-scale effort, comprising over 110 million behavioral measurements, enabled the mapping of social behavior landscapes, defining intricate patterns of interactions and stereotyped actions. Notably, this high-resolution phenotyping revealed autism-specific behavioral alterations, providing a framework for understanding the interaction between social and biological factors underlying neuropsychiatric disorders that critically affect human healthspan and lifespan. Nevertheless, replication of the results in independent large cohorts will be necessary to confirm these perspectives.
Parallel studies have explored the neurobiological circuits regulating ‘social homeostasis’[37], a concept describing an individual’s capacity to assess and modulate the quantity and quality of social contacts to achieve optimal connectivity. Molecular investigations have revealed that the neuropeptide Tac2 orchestrates the behavioral consequences of sustained social isolation stress by acting across multiple cerebral regions[38]. Moreover, recent studies have uncovered a previously uncharacterized hypothalamic circuit explaining the dynamic regulation of social homeostasis[39], while a forebrain-thalamocortical circuit exerts molecular and neural control over social hierarchy[40]. Collectively, these examples underscore the necessity of interdisciplinary approaches integrating social, behavioral, and biomedical factors to develop targeted interventions that promote equity and enhance healthy longevity[41]. This integrative perspective is particularly relevant to exposome research and its implications for aging and age-related diseases.
The exposome concept, initially defined by Wild in 2005 as “the totality of human environmental exposures from conception onwards, complementing the genome”[42], has since expanded to encompass physical, chemical, biological, nutritional, social, economic, and psychological exposures encountered throughout life. Over the past decade, numerous studies have documented the exposome’s role in perturbing somatic and mental health. For example, environmental exposures such as air pollution have been accused of causing the rising incidence of respiratory diseases and lung cancer via mechanisms including epigenetic modifications, oxidative stress, and inflammation[43,44]. Similarly, limited access to green spaces correlates with mental and cardiovascular morbidity[45,46].
Recently, exposome research has been extended to aging studies to evaluate how inadequate exposure to external factors, including social, demographic, and psychological stressors, cumulatively elevate allostatic load, perturbs intrinsic aging processes, and shape aging phenotypes and trajectories[47,48]. Mechanistic studies have linked environmental toxicants—including air and water pollutants, heavy metals, pesticides, and organic solvents—to alterations in the molecular and cellular hallmarks of aging[49,50]. Psychosocial stress, a key mediator of indirect environmental effects, also drives hallmark-specific aging alterations, highlighting the exposome’s broad impact on the molecular basis of aging[51]. However, most epidemiological investigations have focused on single-point exposures, with limited characterization of exposome dynamics across the lifespan.
Argentieri et al. recently conducted a comprehensive integrative analysis of environmental and genetic contributions to age-related morbidity and mortality[52]. They performed an exposome-wide assessment of all-cause mortality in 492,567 UK Biobank participants, followed by an evaluation of associations with proteomic age, aging biomarkers, and age-related multimorbidity, identifying 25 independent exposures significantly linked to premature mortality, accelerated proteomic aging, and age-related disease incidence[52]. Another global study involving 161,981 participants from 40 countries assessed biobehavioral age gaps, defined as the discrepancy between biologically inferred and chronological age, revealing substantial disparities in healthy aging across nations, with European countries exhibiting excellent outcomes, whereas Egypt and South Africa displayed accelerated aging trajectories. These findings further underscore the pivotal influence of socioeconomic and sociopolitical factors, including gender inequality, migration, representation, and democratic processes, on aging trajectories[53]. Data from high-longevity populations[47] reinforce the concept that macro- and micro-environmental exposures, particularly social factors, interact with biological mechanisms to shape human longevity.
Exposome-centered integrative approaches have paved the way for initiatives such as the “Human Exposome Project”[48], which aims to elucidate how external exposures, including social and behavioral factors, interact with internal omics-derived measurements, biomarkers, and physiological assessments to influence age-related conditions, frailty, and functional decline, ultimately modulating healthspan and lifespan. As proposed for Precision Geromedicine[3], the full realization of the Human Exposome Project necessitates advanced artificial intelligence (AI) platforms capable of integrating complex multidimensional data, facilitating the design of personalized interventions to optimize healthspan and lifespan. Complementary initiatives, including the European Human Exposome Network, Exposome Moonshot Forum, NEXUS Network, FAIR Environmental and Health Registry, City of Longevity, EXPOsOMICS in London, and the Exposome Research Hub in Barcelona, aim to continuously monitor internal and external exposures, encompassing social and behavioral dimensions, to improve population health and promote healthier aging.
4. Social Interventions for Improving Health and Longevity
The ongoing advancements in Geroscience and Precision Geromedicine are elucidating the molecular and cellular mechanisms underpinning aging biology, thereby enabling the development of targeted interventions to optimize healthspan and delay the onset of age-related pathologies. Nevertheless, given that the social milieu constitutes a primary determinant of healthy aging and longevity, it is imperative to integrate biologically oriented interventions with strategies addressing the social hallmarks of aging, including the mitigation of systemic social deficiencies and inequities at both population and individual levels. Achieving this integration necessitates progress along two interrelated dimensions: design and implementation of interventions.
4.1 Design of interventions
The initial step involves the conceptualization of the social geroexposome, encompassing macro- and microenvironmental social determinants that causally modulate aging trajectories and longevity outcomes. Prior research has approached this task through three complementary methodologies: natural experiments, field trials, and mechanistic laboratory studies[54]. Natural experiments utilize datasets derived from social adversities in contexts such as natural disasters to interrogate downstream impacts on aging phenotypes and disease susceptibility. Field trials manipulate environmental or social conditions in defined communities to quantify causal improvements in healthspan and quality of life. Laboratory studies employ in vitro and in vivo experimental models to establish mechanistic linkages between social exposures and molecular or cellular aging processes[54]. The integration of these approaches has facilitated the mapping of causal pathways constituting the geroexposome (Figure 2).
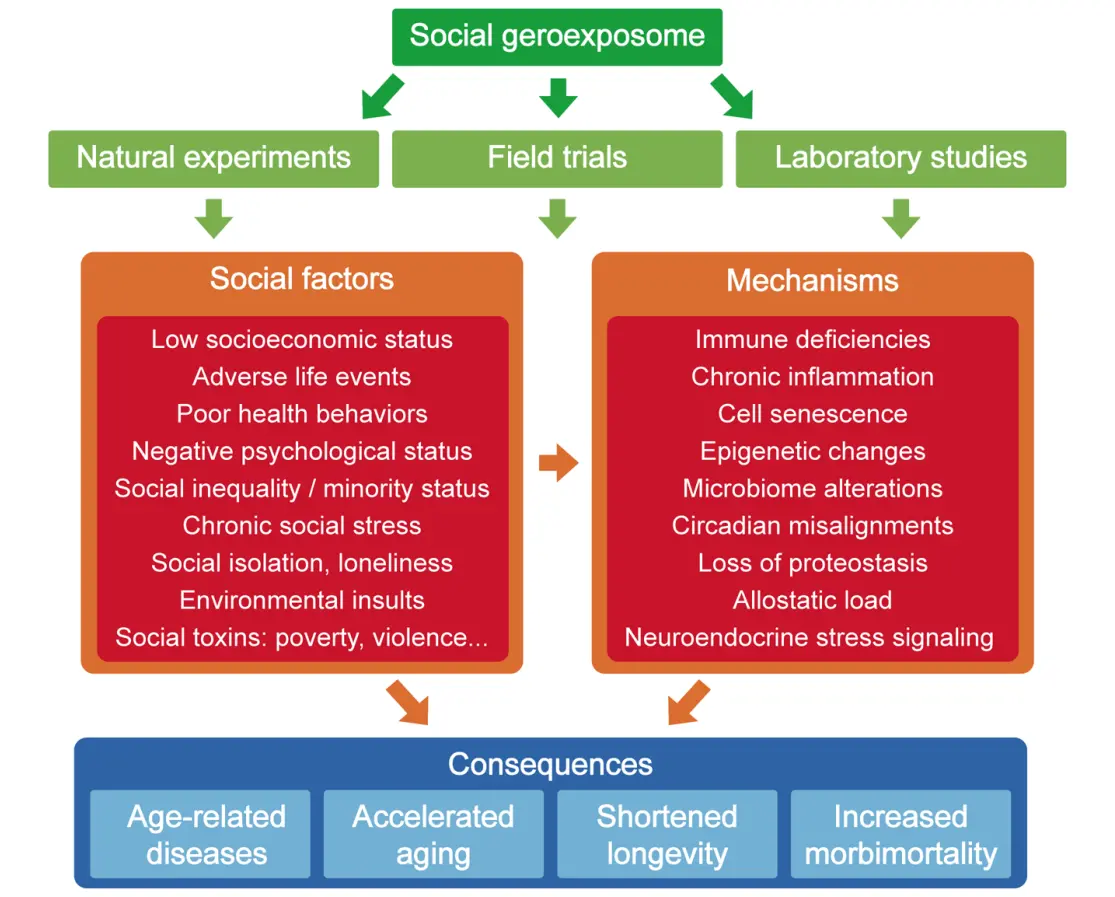
Figure 2. The social geroexposome. The figure indicates the different strategies used for the identification of social factors contributing to aging as well as some illustrative molecular and cellular mechanisms underlying their gerogenic effects. The final outcomes of these social factors and molecular mechanisms for human health and longevity are also indicated.
For instance, Mendelian randomization analyses leveraging genetic instruments associated with the well-being spectrum—spanning life satisfaction, optimism, positive affect, neuroticism and depressive symptoms—while controlling for socioeconomic confounders such as income, occupation and education, have provided robust causal evidence linking social determinants to mental well-being with human longevity[55]. Similarly, meta-analyses encompassing 90 cohort studies have identified social isolation and loneliness as potent risk factors for premature mortality, including all-cause, cardiovascular, and oncological outcomes[56]. Longitudinal studies examining daily fluctuations in loneliness have further demonstrated associations with accelerated biological aging, as measured by biomarkers and self-reported functional decline[57]. Collectively, these findings underscore the critical need for interventions targeting loneliness and social disconnection at both individual and societal levels[58].
Animal model experiments corroborate the causal impact of chronic social stress on lifespan reduction and cardiovascular vulnerability, with concomitant induction of cellular senescence pathways, including p16-mediated senescent cell accumulation, reversible upon targeted senolytic interventions[5,59,60]. These mechanistic insights delineate the molecular conduits through which adverse social environments potentiate aging. Nevertheless, we must emphasize that these studies based on animal models present clear limitations when they are used to analyze the impact of specific biological manipulations on complex social behaviours, or to assess the influence of social determinants on the biological hallmarks of aging. Beyond this cautionary note, the available global analyses seem to converge on the observation that social stress and adversity modulate gerogenic and geroprotective pathways, which, in concert with behavioral and psychological factors, govern aging trajectories. Importantly, while the exposome concept initially emphasized detrimental exposures, positive social interactions serve as critical modulators of human physiology across the lifespan, necessitating the inclusion of pro-social and resilience-enhancing interventions within geroexposome frameworks (Figure 2).
Potential interventions include: enhancement of immune competence; attenuation of systemic inflammation; optimization of gut microbiome diversity as a mediator of immunometabolic resilience[61]; senolytic and senomorphic pharmacological strategies to mitigate sociallyinduced cellular senescence; improvement of sleep quantity and quality; nutritional and physical activity interventions; incorporation of green spaces and walkable infrastructures in urban design; promotion of equitable employment and social participation; mind-body stress management programs; and the mitigation of social toxins, including abuse, violence, inequality, discrimination, and digital addiction (Figure 2). These examples illustrate the potential to translate geroexposome knowledge into actionable strategies for healthspan enhancement, though the dynamic complexity of social exposures poses significant implementation challenges.
4.2 Implementation of interventions
To address this challenge, we must first acknowledge that both the development and implementation of social interventions to improve health and longevity may be very effective without requiring prior understanding of the biological and mechanistic hallmarks of health and aging[3,13,28,29]. Likewise, the implementation of biological interventions to extend healthspan and lifespan can be carried out without significant concern for the social determinants of aging[3,13,28,29]. Based on these premises, if we want to advance in this field, we must try to understand how social interventions can impact underlying biological mechanisms of aging, and reciprocally, how we can contribute to improving the social determinants of aging by addressing the biological mechanisms of this complex process.
To date, and despite compelling evidence for the influence of social and psychological determinants on healthspan and longevity, these factors remain underrepresented within clinical, scientific, and policy frameworks addressing aging[62]. Advances in Geroscience provide molecular targets to modulate gerogenic pathways or enhance geroprotective circuits via pharmacological, lifestyle, or environmental interventions[63,64]. Yet, translating these insights into effective, large-scale social interventions requires the establishment of robust public health policies and community programs that mitigate environmental inequities, promote equitable social determinants, and facilitate healthy aging trajectories (Figure 3).
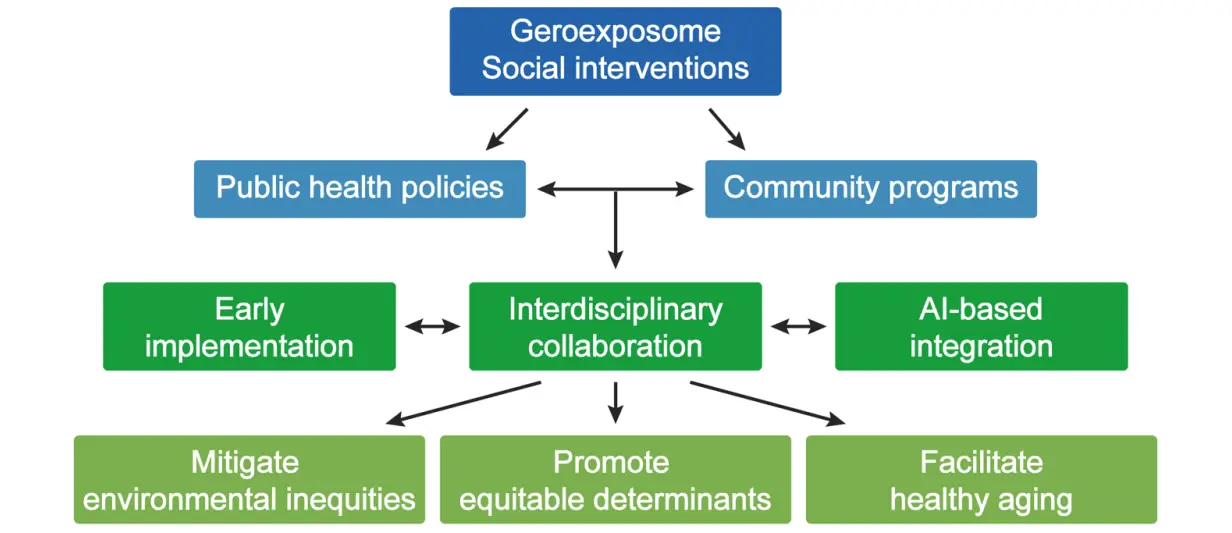
Figure 3. Implementation of interventions targeting the social geroexposome. Summary of the different approaches that can be followed for the precise assessment of the impact of social and environmental interventions on aging trajectories.
Early-life interventions are paramount, as cumulative damage from biological and social hallmarks accelerates molecular deterioration and age-associated morbidity[54]. Interdisciplinary collaboration among healthcare providers, molecular biologists, and social scientists is essential for harmonizing multi-modal datasets encompassing external exposome factors (e.g., environmental pollutants, climate change, social instability) with internal molecular, physiological, and omics-derived data. The integration of generative AI algorithms represents a pivotal tool for synthesizing heterogeneous datasets, identifying predictive patterns, and informing personalized and population-level interventions[54,65,66] (Figure 3). AI-based approaches may further enable quantitative evaluation of intervention efficacy through validated biomarkers of aging, including epigenetic and proteomic aging clocks[67], in conjunction with physiological, cellular, and behavioral metrics such as frailty indices, inflammatory profiles, oxidative stress markers, microbiota composition, endocrine and neurological signaling, and metabolomics.
The fields of Geroscience and Geromedicine, together with those of Gerontology and Geriatrics, are also making significant efforts to develop standardized assessment tools for evaluating social impairment during aging in both humans and animal models. Of particular interest in this regard is the Social Frailty Index, which includes age, gender, and eight different social predictors ranging from neighborhood cleanliness to experiences of being treated with less courtesy or respect[68]. Recent studies have shown that this index accurately stratifies risk among older adults, provides superior predictive information compared with other existing tools, and may prove highly valuable in clinical, epidemiological, and experimental studies focused on assessing interventions relevant to Geroscience and Geromedicine[68].
Taken together, this comprehensive, multidisciplinary evaluation framework—integrating AI-driven methodologies, population health studies, and detailed molecular and cellular multi-omic analyses—will enable a more precise assessment of the impact of social and environmental interventions on individual aging trajectories.
5. Conclusions and Perspectives
Aging represents a dynamic and multifaceted biological phenomenon, orchestrated by the cumulative interplay of molecular, cellular, environmental, and psychosocial determinants. The progressive elaboration of the “hallmarks of aging” framework has provided an invaluable conceptual scaffold for disentangling the extraordinary complexity of this process[69]. Its current expansion to encompass psychosocial isolation and mental illness reflects a decisive shift toward a more integrative understanding of organismal senescence that transcends the boundaries of purely molecular biology. Indeed, the inclusion of social and exposome-related aging hallmarks highlights the profound and often underestimated influence of socioeconomic disadvantage, chronic stress, behavioral adversity, and structural inequities on healthspan trajectories and longevity outcomes.
These insights form the intellectual foundation of Precision Geromedicine, an emerging paradigm that seeks to interlace multi-omic datasets, exposomic measurements, and clinical biomarkers with social and behavioral parameters in order to delineate individualized risk and resilience profiles. This integrative approach, leveraging advanced AI and systems-level modeling, aspires not only to unravel the mechanistic determinants of aging but also to facilitate the rational design of targeted interventions across the lifespan. Achieving this vision necessitates unprecedented collaboration among molecular biologists, geroscientists, social scientists, healthcare providers, epidemiologists, data scientists, and policymakers. Likewise, there is an urgent need to advance toward the deeper integration of the biological and social hallmarks of aging while acknowledging the current limitations imposed by the available in vivo experimental models, which are often insufficient to fully capture the bidirectional impact of complex social behaviors on biological aging—and vice versa. Similar considerations apply to the functional validation of the potential relevance of genetic variants in relation to various social markers, as well as to their ultimate impact on the dynamic and highly heterogeneous biological processes underlying human aging.
Ultimately, the convergence of biological and societal interventions offers a transformative opportunity to extend healthspan, mitigate age-associated morbidities, and reduce inequities in aging trajectories. A holistic and interdisciplinary approach to aging research is therefore indispensable for fostering equitable and sustainable strategies that optimize human longevity in an increasingly complex global landscape.
Acknowledgements
We are very grateful to Drs. Yaiza Español and David Roiz for helpful comments to the manuscript. We also acknowledge the insightful and constructive comments of the experts who reviewed our manuscript.
Authors contribution
López-Otin C: Designed the manuscript, wrote the initial draft.
Kroemer G: Designed the manuscript.
All authors have given approval to the final version of the manuscript.
Conflicts of interest
Guido Kroemer serves as the Editor-in-Chief and Carlos López-Otín is an Editorial Board Member of Geromedicine. The authors declare no other conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
None.
Copyright
© The Author(s) 2025.
References
-
1. Muscedere J, Shorey CL, Duque G, Kim P, Lorbergs AL, McGlory C, et al. Advancing geroscience research-a scoping review of regulatory environments for gerotherapeutics. J Nutr Health Aging. 2025;29(9):100637.[DOI]
-
2. Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: Linking aging to chronic disease. Cell. 2014;159(4):709-713.[DOI]
-
3. Kroemer G, Maier AB, Cuervo AM, Gladyshev VN, Ferrucci L, Gorbunova V, et al. From geroscience to precision geromedicine: Understanding and managing aging. Cell. 2025;188(8):2043-2062.[DOI]
-
4. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194-1217.[DOI]
-
5. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186(2):243-278.[DOI]
-
6. Mavrogonatou E, Papadopoulou A, Pratsinis H, Kletsas D. Senescence-associated alterations in the extracellular matrix: Deciphering their role in the regulation of cellular function. Am J Physiol Cell Physiol. 2023;325(3):C633-C647.[DOI]
-
7. Zhang H, Tsui CK, Garcia G, Joe LK, Wu H, Maruichi A, et al. The extracellular matrix integrates mitochondrial homeostasis. Cell. 2024;187(16):4289-4304.[DOI]
-
8. Selman M, Pardo A. Fibroageing: An ageing pathological feature driven by dysregulated extracellular matrix-cell mechanobiology. Ageing Res Rev. 2021;70:101393.[DOI]
-
9. Barakat N, Jangir H, Gallo L, Grillo M, Guo X, Hickman J. Inhibition of metalloproteinases extends longevity and function of in vitro human ipsc-derived skeletal muscle. Biomedicines. 2024;12(4):856.[DOI]
-
10. Zhang Z, Tian X, Lu JY, Boit K, Ablaeva J, Zakusilo FT, et al. Increased hyaluronan by naked mole-rat has2 improves healthspan in mice. Nature. 2023;621(7977):196-205.[DOI]
-
11. Kivimäki M, Bartolomucci A, Kawachi I. The multiple roles of life stress in metabolic disorders. Nat Rev Endocrinol. 2023;19(1):10-27.[DOI]
-
12. Snyder-Mackler N, Burger JR, Gaydosh L, Belsky DW, Noppert GA, Campos FA, et al. Social determinants of health and survival in humans and other animals. Science. 2020;368(6493):eaax9553.[DOI]
-
13. Crimmins EM. Social hallmarks of aging: Suggestions for geroscience research. Ageing Res Rev. 2020;63:101136.[DOI]
-
14. Bhutta ZA, Bhavnani S, Betancourt TS, Tomlinson M, Patel V. Adverse childhood experiences and lifelong health. Nat Med. 2023;29(7):1639-1648.[DOI]
-
15. Ridley M, Rao G, Schilbach F, Patel V. Poverty, depression, and anxiety: Causal evidence and mechanisms. Science. 2020;370(6522):eaay0214.[DOI]
-
16. Chen M, Gao Q, Zhu X, Zhang J, Xia R, Zhang Q. Dietary inflammatory index and depressive symptoms as mediators between social disadvantage and accelerated phenotypic aging. J Affect Disord. 2025;391:119995.[DOI]
-
17. Holt-Lunstad J. Loneliness and social isolation as risk factors: The power of social connection in prevention. Am J Lifestyle Med. 2021;15(5):567-573.[DOI]
-
18. López-Otín C, Kroemer G. The missing hallmark of health: Psychosocial adaptation. Cell Stress. 2024;8:21-50.[DOI]
-
19. Dale W, Klepin HD, Williams GR, Alibhai SMH, Bergerot C, Brintzenhofeszoc K, et al. Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: Asco guideline update. J Clin Oncol. 2023;41(26):4293-4312.[DOI]
-
20. Hall MB, Willis DE, Rodriguez EL, Schwarz JM. Maternal immune activation as an epidemiological risk factor for neurodevelopmental disorders: Considerations of timing, severity, individual differences, and sex in human and rodent studies. Front Neurosci. 2023;17:1135559.[DOI]
-
21. Berkman LF, Syme SL. Social networks, host resistance, and mortality: A nine-year follow-up study of alameda county residents. Am J Epidemiol. 1979;109(2):186-204.[DOI]
-
22. Pitkala KH, Routasalo P, Kautiainen H, Tilvis RS. Effects of psychosocial group rehabilitation on health, use of health care services, and mortality of older persons suffering from loneliness: A randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64(7):792-800.[DOI]
-
23. Shen C, Wang DN, Gao XX, Zhao R, Dong C, Gu ZF, et al. A study on the impact of the number of family generations on intergenerational support for centenarians: A study in a chinese “longevity city”. Psychogeriatrics. 2023;23(6):908-917.[DOI]
-
24. Díaz-Del Cerro E, Ceprián N, Félix J, De la Fuente M. A short social interaction between adult and old mice improves the homeostatic systems and increases healthy longevity. Exp Gerontol. 2022;158:111653.[DOI]
-
25. Zhu P, Liu W, Zhang X, Li M, Liu G, Yu Y, et al. Correlated evolution of social organization and lifespan in mammals. Nat Commun. 2023;14(1):372.[DOI]
-
26. Legaz A, Altschuler F, Gonzalez-Gomez R, Hernández H, Baez S, Migeot J, et al. Structural inequality linked to brain volume and network dynamics in aging and dementia across the americas. Nat Aging. 2025;5(2):259-274.[DOI]
-
27. Kivimäki M, Pentti J, Frank P, Liu F, Blake A, Nyberg ST, et al. Social disadvantage accelerates aging. Nat Med. 2025;31(5):1635-1643.[DOI]
-
28. López-Otín C, Kroemer G. Hallmarks of health. Cell. 2021;184(1):33-63.[DOI]
-
29. Holt-Lunstad J. Social connection as a critical factor for mental and physical health: Evidence, trends, challenges, and future implications. World Psychiatry. 2024;23(3):312-332.[DOI]
-
30. Gellert P, Alonso-Perez E. Psychosocial and biological pathways to aging : The role(s) of the behavioral and social sciences in geroscience. Z Gerontol Geriatr. 2024;57(5):365-370.[DOI]
-
31. Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: macarthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98(8):4770-4775.[DOI]
-
32. Kim GR, Jee SH, Pikhart H. Role of allostatic load and health behaviours in explaining socioeconomic disparities in mortality: A structural equation modelling approach. J Epidemiol Community Health. 2018;72(6):545-551.[DOI]
-
33. Mira R, Newton JT, Sabbah W. The longitudinal relationship between allostatic load and multimorbidity among older americans. Geriatrics. 2025;10(4):84.[DOI]
-
34. Bobba-Alves N, Sturm G, Lin J, Ware SA, Karan KR, Monzel AS, et al. Cellular allostatic load is linked to increased energy expenditure and accelerated biological aging. Psychoneuroendocrinology. 2023;155:106322.[DOI]
-
35. Wu XR, Yang L, Wu BS, Liu WS, Deng YT, Kang JJ, et al. Exome sequencing identifies genes for socioeconomic status in 350,770 individuals. Proc Natl Acad Sci U S A. 2025;122(2):e2414018122.[DOI]
-
36. Klibaite U, Li T, Aldarondo D, Akoad JF, Ölveczky BP, Dunn TW. Mapping the landscape of social behavior. Cell. 2025;188(8):2249-2266.[DOI]
-
37. Lee CR, Chen A, Tye KM. The neural circuitry of social homeostasis: Consequences of acute versus chronic social isolation. Cell. 2021;184(6):1500-1516.[DOI]
-
38. Zelikowsky M, Hui M, Karigo T, Choe A, Yang B, Blanco MR, et al. The neuropeptide tac2 controls a distributed brain state induced by chronic social isolation stress. Cell. 2018;173(5):1265-1279.[DOI]
-
39. Liu D, Rahman M, Johnson A, Amo R, Tsutsui-Kimura I, Sullivan ZA, et al. A hypothalamic circuit underlying the dynamic control of social homeostasis. Nature. 2025;640(8060):1000-1010.[DOI]
-
40. Nelson AC, Kapoor V, Vaughn E, Gnanasegaram JA, Rubinstein ND, Talay M, et al. Molecular and neural control of social hierarchy by a forebrain-thalamocortical circuit. Cell. 2025;188(20):5535-5554.[DOI]
-
41. Baumer Y, Powell-Wiley TM. Interdisciplinary approaches are fundamental to decode the biology of adversity. Cell. 2021;184(11):2797-2801.[DOI]
-
42. Wild CP. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847-1850.[DOI]
-
43. McAllister BJ, Malhotra Mukhtyar R, Cai S, Brown K, Lock S, Khan S. Relationship between household air pollution and lung cancer in never smokers in high-income countries: A systematic review. BMJ Open. 2025;15(6):e093870.[DOI]
-
44. Zhang ZQ, Li JY, Guo Q, Li YL, Bao YW, Song YQ, et al. Association between air pollution and allergic upper respiratory diseases: A meta-analysis. Eur Respir Rev. 2025;34(176):240266.[DOI]
-
45. Bressane A, Silva MB, Goulart APG, Medeiros LC de C. Understanding how green space naturalness impacts public well-being: Prospects for designing healthier cities. Int J Environ Res Public Health. 2024;21(5):585.[DOI]
-
46. Lu Q, Lian C, Chen X. Green space is associated with new-onset stroke among chinese middle-aged and older adults: Data from china health and retirement longitudinal study (CHARLS). Front Public Health. 2024;12:1424510.[DOI]
-
47. Aliberti SM, Capunzo M. The power of environment: A comprehensive review of the exposome’s role in healthy aging, longevity, and preventive medicine-lessons from blue zones and cilento. Nutrients. 2025;17(4):722.[DOI]
-
48. Woods T, Palmarini N, Corner L, Barzilai N, Maier AB, Sagner M, et al. Cities, communities and clinics can be testbeds for human exposome and aging research. Nat Med. 2025;31(4):1066-1068.[DOI]
-
49. Pandics T, Major D, Fazekas-Pongor V, Szarvas Z, Peterfi A, Mukli P, et al. Exposome and unhealthy aging: Environmental drivers from air pollution to occupational exposures. Geroscience. 2023;45(6):3381-3408.[DOI]
-
50. Kalia V, Belsky DW, Baccarelli AA, Miller GW. An exposomic framework to uncover environmental drivers of aging. Exposome. 2022;2(1):osac002.[DOI]
-
51. Polsky LR, Rentscher KE, Carroll JE. Stress-induced biological aging: A review and guide for research priorities. Brain Behav Immun. 2022;104:97-109.[DOI]
-
52. Argentieri MA, Amin N, Nevado-Holgado AJ, Sproviero W, Collister JA, Keestra SM, et al. Integrating the environmental and genetic architectures of aging and mortality. Nat Med. 2025;31(3):1016-1025.[DOI]
-
53. Hernandez H, Santamaria-Garcia H, Moguilner S, Farina FR, Legaz A, Prado P, et al. The exposome of healthy and accelerated aging across 40 countries. Nat Med. 2025;31(9):3089-3100.[DOI]
-
54. Belsky DW, Baccarelli AA. To promote healthy aging, focus on the environment. Nat Aging. 2023;3(11):1334-1344.[DOI]
-
55. Ye CJ, Liu D, Chen ML, Kong LJ, Dou C, Wang YY, et al. Mendelian randomization evidence for the causal effect of mental well-being on healthy aging. Nat Hum Behav. 2024;8(9):1798-1809.[DOI]
-
56. Wang F, Gao Y, Han Z, Yu Y, Long Z, Jiang X, et al. A systematic review and meta-analysis of 90 cohort studies of social isolation, loneliness and mortality. Nat Hum Behav. 2023;7(8):1307-1319.[DOI]
-
57. Wilson SJ, Koffer RE. Lonely days: Linking day-to-day loneliness to biological and functional aging. Health Psychol. 2025;44(5):446-455.[DOI]
-
58. Jeste DV, Lee EE, Cacioppo S. Battling the modern behavioral epidemic of loneliness: Suggestions for research and interventions. JAMA Psychiatry. 2020;77(6):553-554.[DOI]
-
59. Razzoli M, Nyuyki-Dufe K, Gurney A, Erickson C, McCallum J, Spielman N, et al. Social stress shortens lifespan in mice. Aging Cell. 2018;17(4):e12778.[DOI]
-
60. Lyons CE, Pallais JP, McGonigle S, Mansk RP, Collinge CW, Yousefzadeh MJ, et al. Chronic social stress induces p16-mediated senescent cell accumulation in mice. Nat Aging. 2025;5(1):48-64.[DOI]
-
61. Benner C, Cesari M, Sadana R. Microbiological foundations to optimise intrinsic capacity and promote healthy ageing: An integration into the life course approach. Aging Cell. 2025;24(8):e70146.[DOI]
-
62. Faria M, Ganz A, Galkin F, Zhavoronkov A, Snyder M. Psychogenic aging: A novel prospect to integrate psychobiological hallmarks of aging. Transl Psychiatry. 2024;14(1):226.[DOI]
-
63. Epel ES. The geroscience agenda: Toxic stress, hormetic stress, and the rate of aging. Ageing Res Rev. 2020;63:101167.[DOI]
-
64. López-Otín C, Maier AB, Kroemer G. Gerogenes and gerosuppression: The pillars of precision geromedicine. Cell Res. 2024;34(7):463-466.[DOI]
-
65. Capraro V, Lentsch A, Acemoglu D, Akgun S, Akhmedova A, Bilancini E, et al. The impact of generative artificial intelligence on socioeconomic inequalities and policy making. PNAS Nexus. 2024;3(6):191.[DOI]
-
66. Hartung T. How AI can deliver the human exposome project. Nat Med. 2025;31(6):1738.[DOI]
-
67. Kusters CDJ, Horvath S. Quantification of epigenetic aging in public health. Annu Rev Public Health. 2025;46(1):91-110.[DOI]
-
68. Shah SJ, Oreper S, Jeon SY, Boscardin WJ, Fang MC, Covinsky KE. Social frailty index: Development and validation of an index of social attributes predictive of mortality in older adults. Proc Natl Acad Sci USA. 2023;120(7):e2209414120.[DOI]
-
69. Tartiere AG, Freije JMP, López-Otín C. The hallmarks of aging as a conceptual framework for health and longevity research. Front Aging. 2024;5:1334261.[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




