Abstract
The treatment of early invasive breast cancer in older patients poses unique challenges due to the distinct biological, clinical, and psychosocial complexities associated with aging. As the population of breast cancer patients aged 70 years and older continues to grow, their persistent underrepresentation in clinical trials remains a major obstacle to evidence-based treatment decision-making. To support the development of a more effective, personalized, and patient-centered approach to systemic therapy, this review outlines the biological features of breast cancer in older women, synthesizes current evidence on neoadjuvant and adjuvant systemic therapies, and discusses strategies for individualized treatment decision-making. Key recommendations include the use of hormonal therapy as the standard of care for hormone receptor positive breast cancer, neoadjuvant therapy primarily when tumor downstaging is desired, and chemotherapy or anti-human epidermal growth factor receptor 2 therapy for relatively fit older patients with high-risk subtypes. Additionally, bisphosphonates may help preserve bone health and reduce recurrence risk. Novel targeted therapies such as cyclin-dependent kinase 4 and 6 inhibitors and immune checkpoint inhibitors show promise, though further studies are needed to confirm their safety and efficacy in older populations. Comprehensive geriatric assessments are essential for identifying patient frailty and vulnerabilities, while predictive tools such as the Cancer and Aging Research Group Breast Cancer model can help assess toxicity risk. In this population, competing risks of non-cancer-related mortality may reduce the absolute benefit of systemic treatment. For patients with elevated risks of other-cause mortality, the potential survival benefit of cancer therapy may be negligible. Predictive models that account for competing mortality, such as the PORTRET tool, can facilitate personalized and shared decision-making.
Keywords
1. Introduction
Breast cancer is the most commonly diagnosed malignancy among women, with approximately 30% of cases occurring in individuals aged 70 years or older at the time of diagnosis[1]. As the global population continues to age, the number of older patients with breast cancer is expected to increase substantially.
Treating early-stage breast cancer in older patients presents several challenges. First, this population exhibits wide variability in overall health status, ranging from robust individuals capable of tolerating standard therapies to frail patients who are more susceptible to adverse outcomes and early discontinuation of treatment[2]. Age-related physiological changes, including decreased organ function and altered drug metabolism, can further compromise treatment tolerability[3]. Second, older adults often have multiple comorbidities and reduced physical fitness, both of which increase the risk of non-cancer-related mortality and may attenuate the therapeutic benefits of breast cancer treatment[4]. Third, many older patients place greater value on maintaining independence, cognitive function, and quality of life rather than pursuing maximal survival gains[5]. These factors collectively highlight the importance of a personalized treatment approach that carefully weighs the potential benefits of therapy against the associated risks of toxicity.
Despite the growing population of older breast cancer patients and the unique biological, clinical, and psychosocial complexities they present, this group remains significantly underrepresented in clinical trials. This is particularly evident in studies evaluating systemic neoadjuvant and adjuvant therapies[6,7]. As a result, there is a paucity of robust evidence to guide treatment recommendations for this demographic[6,7].
Consequently, current clinical guidelines offer limited direction on the use of systemic neoadjuvant and adjuvant therapies in older breast cancer patients. For example, the European Society for Medical Oncology (ESMO) advocates for treatment decisions based on biological rather than chronological age. Less intensive regimens are recommended for frail individuals, while fit patients may be considered for standard multidrug therapies when appropriate[8]. ESMO also highlights the importance of comprehensive geriatric assessment and recommends the use of tools such as the G8 screening instrument to identify patients who require further evaluation[8]. Similarly, the Dutch Federation of Medical Specialists guidelines recommend adjuvant chemotherapy for patients aged 70 years or older only when the expected clinical benefit is substantial and the patient is in good general condition[9]. However, both sets of guidelines lack precise definitions of what constitutes “substantial benefit” or “good condition”.
This review seeks to support the development of more effective, personalized, and patient-centered systemic treatment strategies for older adults with early invasive breast cancer. It begins by outlining the biological characteristics of breast cancer in older women, then synthesizes current evidence on neoadjuvant and adjuvant systemic therapies, and finally explores strategies to tailor treatment decisions to the individual patient.
2. Biological Characteristics of Breast Cancer in Older Patients
Breast cancer in older women often demonstrates more favorable biological features[10]. Tumors in this population are frequently hormone receptor-positive (HR+). A combined analysis of the Age Gap UK and CLIMB NL studies reported that 87.7% of tumors were estrogen receptor-positive (ER+) and 69.1% were progesterone receptor-positive (PR+), indicating a high likelihood of response to hormonal therapy[11]. These tumors also tend to present as mucinous carcinomas, a histological subtype associated with a more favorable prognosis[12]. Moreover, human epidermal growth factor receptor 2 (HER2) expression is less common in older women, with only 12.3% of cases being HER2-positive (HER2+), which is advantageous given the typically aggressive nature of HER2+ cancers[11,13]. Lower Ki67 expression, a marker of cellular proliferation and tumor aggressiveness, is also more frequently observed in this age group. Specifically, Ki67 expression greater than 30% is reported in only 17.9% of older women, compared to 20.8% in middle-aged and 35.0% in younger women[10,13]. Additionally, older women are less likely to develop triple negative (HR negative, HER2 negative) breast cancer, a highly aggressive subtype that is generally more difficult to treat[10].
The risk and timing of breast cancer recurrence vary according to tumor biology[14,15]. In HR+ disease, the recurrence risk gradually increases over the first three years and then stabilizes[16]. In contrast, HR negative (HR-) tumors show a higher recurrence risk during the initial two years, which declines between years three and four, and subsequently rises again, eventually matching the recurrence risk observed in HR+ disease by year eight[16]. Survival after recurrence (SAR) also differs by subtype[17]. Patients with HR+ HER2- disease have the longest median SAR (80 months), followed by HR+ HER2+ (67 months), HR- HER2+ (49 months), and HR- HER2- disease, which has the shortest median SAR (23 months)[17]. In HR+ disease, where recurrence tends to occur later and SAR is longer, the potential benefits of adjuvant therapy must be carefully weighed against the competing risk of mortality from non-cancer-related causes.
3. Systemic Treatments for Older Patients with Early Invasive Breast Cancer
Surgery, often combined with radiotherapy, constitutes a fundamental component of breast cancer treatment, particularly in older patients. Systemic adjuvant therapies aim to eradicate any residual cancer cells, thereby reducing the risk of recurrence and improving long-term survival[18]. Adjuvant systemic treatment or a portion thereof can also be administered prior to surgery in the neoadjuvant setting. This strategy further aims to downstage the tumor, making breast-conserving surgery (BCS) a feasible alternative to mastectomy[19,20], or allowing for limited axillary treatment[18,19]. Various types of systemic treatments exist, including hormonal therapy, chemotherapy, and anti-HER2 therapy. More recently, targeted therapies such as cyclin-dependent kinase 4/6 (CDK4/6) inhibitors and immune checkpoint inhibitors have demonstrated efficacy in the adjuvant setting, while others, including phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) inhibitors, are currently being explored for early-stage breast cancer.
3.1 Hormonal therapy
Hormonal therapy is commonly employed in older breast cancer patients, as most tumors are HR+[21]. It targets estrogen and/or progesterone signaling pathways that drive the growth and proliferation of HR+ breast cancer cells[22]. Hormonal therapy may be used as a primary treatment, in the neoadjuvant setting, or as adjuvant therapy following surgery. Common agents include tamoxifen and aromatase inhibitors (AIs), each with distinct benefits and risk profiles[23].
3.1.1 Adjuvant hormonal therapy
Meta-analyses by the Early Breast Cancer Trialists’ Collaborative Group demonstrated that adjuvant hormonal therapy reduces the annual breast cancer mortality rate by 31% in women with HR+ disease, largely independent of age, chemotherapy use, progesterone receptor status, or other tumor characteristics[24]. Although few women over 70 years old were included in these early trials[24], a review of 483 older women (≥ 70 years) with stage I-III HR+ disease confirmed a significant survival benefit associated with adjuvant hormonal therapy. This treatment was linked to improved overall survival (OS) (hazard ratio [HR] = 0.44; p = 0.004) and disease-free survival (HR = 0.42; p < 0.01)[25].
However, patients with low-risk, early-stage disease may derive limited benefit. A study of 224 women aged ≥ 65 years with T1N0M0 tumors found no significant differences in 10-year contralateral relapse rates or OS with tamoxifen treatment[26]. Similarly, a Danish cohort study involving 3,197 women with node-negative HR+ breast cancer reported that those aged 60-74 years with small tumors (≤ 10 mm) and low-grade histopathological features (grade 1 ductal carcinoma or grade 1-2 lobular carcinoma) did not experience increased mortality risk when systemic adjuvant therapy was omitted (adjusted relative risk [RR] = 1.02; 95% confidence interval [CI], 0.89-1.16)[27]. In such low-risk disease, the modest absolute benefit of hormonal therapy in reducing breast cancer recurrence is counterbalanced by mortality from other causes.
3.1.2 Neoadjuvant hormonal therapy
Neoadjuvant hormonal therapy (NET) is increasingly employed in older patients with HR+ breast cancer to reduce tumor size prior to surgery and facilitate BCS[20]. However, neoadjuvant studies have generally included only a limited number of older patients, which may limit the generalizability of these findings to this population.
The limited studies focusing specifically on older women have reported clinical response rates ranging from 48% to 70%[28-30]. Better responses are observed in patients exhibiting strong hormone receptor positivity, a low Ki67 proliferation index, and HER2 negativity[28-30]. AIs generally yield superior responses compared to tamoxifen[28]. Response to NET may continue to improve for up to 12 months, suggesting that longer treatment durations could enhance outcomes[31]. Across studies, conversion rates from mastectomy to breast-conserving therapy average 30.2%, while pathological complete response (pCR) rates remain low, typically below 23% in the breast[32] and approximately 9% in lymph nodes[19].
Approximately 9% of patients may experience disease progression during NET, underscoring the importance of regular clinical monitoring and early detection to enable timely intervention[20]. Furthermore, response assessment using magnetic resonance imaging (MRI) can be challenging due to the “cookie crumble” effect, where the tumor fragments into smaller pieces, complicating accurate evaluation[32].
3.1.3 Safety and adherence
Safety is a critical consideration when prescribing hormonal therapy for older patients. Although studies have reported grade 3-4 toxicity rates of only 0-9%[20,28],these data primarily derive from cohorts of relatively young and very fit older individuals. Therefore, toxicity rates are likely higher in older patients with comorbidities[33]. Tamoxifen has been associated with increased risks of thromboembolic events, cerebrovascular accidents, and uterine cancer[34,35]. AIs elevate the risk of osteoporosis, fractures, and cardiovascular events[34,35]. Consequently, adjuvant bisphosphonates are recommended to preserve or improve bone health, particularly during AI therapy[36]. Bisphosphonates also confer a survival benefit in postmenopausal women with early breast cancer, irrespective of breast cancer subtype or systemic treatment[37,38]. Despite some safety concerns, studies in older women indicate that adjuvant hormonal therapy is not associated with cognitive decline, functional impairment, or reduced quality of life[39,40].
Adherence to hormonal therapy remains a challenge among older patients. One study reported that 36% of older women discontinued treatment within two years[39]. However, those with higher-risk tumors were more likely to maintain therapy[39]. Notably, no geriatric factors have been consistently linked to early treatment discontinuation[39].
3.1.4 CDK4/6 inhibitors
CDK4/6 inhibitors exert their effect by preventing phosphorylation of the retinoblastoma (Rb) protein, thereby arresting cell cycle progression and inhibiting tumor growth. Recent clinical trials in early-stage disease have demonstrated that combining CDK4/6 inhibitors with hormonal therapy improves outcomes in HR+ HER2- breast cancer.
In the monarchE trial involving 5,637 high-risk, early-stage patients, the addition of abemaciclib to hormonal therapy significantly improved outcomes[41]. At a median follow-up of 54 months, abemaciclib was associated with hazard ratios of 0.680 (95% CI, 0.599 to 0.772) for invasive disease-free survival (IDFS) and 0.675 (95% CI, 0.588 to 0.774) for distant relapse-free survival. However, OS was not significantly increased[41]. Only a small subgroup of patients were aged ≥ 65 years; within this subgroup, the IDFS benefit was less definitive (HR 0.767; 95% CI, 0.556 to 1.059)[41].
In the NATALEE trial including 5,101 patients with stage II-III breast cancer, ribociclib combined with hormonal therapy resulted in a three-year IDFS rate of 90.4%, compared to 87.1% in the hormonal therapy-alone arm (HR = 0.75; 95% CI, 0.62 to 0.91; p = 0.0014)[42]. Benefits were consistent across predefined subgroups[42]. Overall survival data are not yet mature.
3.2 Chemotherapy
Chemotherapy employs cytotoxic agents to target rapidly proliferating cells. These agents interfere with critical cellular processes including DNA replication, RNA synthesis, and mitotic division, ultimately inducing cancer cell death[43]. Chemotherapy can be administered in the neoadjuvant setting or as adjuvant therapy following surgery to reduce the risk of recurrence. Standard breast cancer chemotherapy regimens include anthracycline-based and taxane-based treatments, with sequential regimens recommended for very fit patients. Adjuvant capecitabine may be considered for fit older women with triple negative breast cancer who have residual disease following neoadjuvant chemotherapy.
3.2.1 Adjuvant chemotherapy
A meta-analysis of randomized controlled trials (RCTs) investigating contemporary chemotherapy regimens for early breast cancer demonstrated an age-dependent effect[24]. Six months of anthracycline-based polychemotherapy reduced breast cancer mortality by approximately 38% in women under 50 and by 20% in women aged 50-69, irrespective of ER status, hormonal treatment, nodal status, or other tumor characteristics[24]. Extending the number of chemotherapy cycles, adding taxanes to anthracycline-based regimens, and employing dose-dense schedules further decreased breast cancer mortality rates[44]. Unfortunately, very few women aged 70 or older participated in these studies.
A limited number of clinical trials have specifically examined chemotherapy in older women, with most focusing on comparisons among chemotherapy regimens rather than evaluating chemotherapy versus no chemotherapy[24,44,45].
The randomized ASTER 70s trial targeted HR+ HER2- early-stage breast cancer patients aged 70 years or older with a high genomic grade index (GGI)[46]. The GGI is a measure of tumor aggressiveness based on genetic markers. Patients were randomized to receive chemo-hormonal therapy or hormonal therapy alone. After a median follow-up of 5.8 years, the 4-year OS rates were 90.5% in the chemo-hormonal group versus 89.7% in the hormonal therapy-alone group; this difference was not statistically significant (HR = 0.85; p = 0.2538)[47]. However, in this cohort of ER+ women, the follow-up period may be too short to capture the majority of recurrences. Furthermore, 20.5% of patients assigned to the chemo-hormonal group did not receive chemotherapy, likely attenuating the observed effects and contributing to the lack of significance in the intent-to-treat analysis[46,47]. The per-protocol analysis showed some improvement in 4-year OS in the chemo-hormonal therapy group (91%) compared with the hormonal therapy-alone group (89.3%) (HR = 0.73; p = 0.03)[47].
The randomized ICE study enrolled 1,409 women aged 65 years or older with high-risk early breast cancer and found no survival benefit from adjuvant capecitabine[48]. Unfortunately, further RCTs specifically including older women with early breast cancer and HR- disease are lacking.
Two retrospective cohort studies using the Surveillance, Epidemiology, and End Results database demonstrated significant survival benefits associated with chemotherapy in women over 70 years old with HR- breast cancer and either node-positive disease or other high-risk features[49,50].One study reported that adjuvant chemotherapy was associated with approximately a 16% reduction in all-cause mortality among older women with HR- breast cancer[49]. The other found that chemotherapy improved overall survival only in women with lymph node–positive HR- breast cancer (HR = 0.65; 95% CI, 0.52 to 0.82)[50]. However, caution is warranted when interpreting observational data due to selection biases that multivariate models cannot fully address[50]. Despite statistical adjustments, unmeasured confounders remain, underscoring the need for validation through randomized trials.
These findings suggest that the survival benefit of adding chemotherapy to hormonal therapy may be modest in older patients with HR+ HER2- breast cancer. In contrast, in patients with HR- breast cancer and high-risk subtypes, the survival benefit is more pronounced[23,24,49,50]. This difference may partly be explained by the higher short-term recurrence risk in HR- disease, which reduces the likelihood of death from other causes before recurrence and thereby results in a greater absolute benefit from chemotherapy.
3.2.2 Neoadjuvant chemotherapy
Neoadjuvant chemotherapy (NAC) not only downstages tumors but also enables assessment of treatment response, which can subsequently inform adjuvant therapy decisions. Therefore, when chemotherapy is deemed likely to improve outcomes for an older patient, neoadjuvant administration is often preferred.
Although data on NAC efficacy in older patients remain limited, response rates appear comparable to those in younger patients, provided the tumor subtype is the same. A study comparing NAC downstaging rates and tolerability between women aged 50-69 and those aged 70 or older found that the older cohort received less anthracycline-based therapy and was less likely to complete the prescribed treatment regimen. Nevertheless, they achieved high rates of breast and axillary downstaging comparable to younger patients[51]. Conversion rates to BCS eligibility were 72% among patients aged 70 and older[51]. Nodal pCR rates were 43% in the older group compared to 42% in the younger group (p > 0.9)[51].
Receptor subtype significantly influences nodal pCR outcomes following NAC. HER2- and triple-negative breast cancers are more likely to achieve nodal pCR compared to hormone HR+ HER2- cancers (HER2+ OR = 7.31; 95% CI, 4.10 to 13.4; triple-negative OR = 2.04; 95% CI, 1.11 to 3.80; p < 0.001)[51]. These findings align with another study reporting distinct pCR rates post-NAC: 10% for luminal A (ER+, PR+), 46.9% for triple-negative, and 72.7% for HER2+ breast cancers[52].
A study comparing NET with NAC in postmenopausal HR+ breast cancer patients found similar pCR outcomes between the two treatments[20]. The clinical objective response rate was 64% in both groups[20]. Disease progression rates were identical at 9% for each group. Conversion rates to BCS were not significantly higher in the NET group (33% vs. 24%)[20].
3.2.3 Safety
In older patients, chemotherapy carries a high risk of severe toxicity, with common complications including acute kidney injury, electrolyte imbalances, and cardiac and hematological toxicities[23,53]. Studies report that approximately 46% to 53% of older adults with cancer experience severe (grade 3-5) toxicities during chemotherapy[54,55].
Such toxicities may lead to treatment delays or modifications that could compromise overall treatment efficacy. Examples of treatment modifications include switching to conventional rather than dose-dense chemotherapy schedules, reducing the number of cycles (e.g., four instead of six), or limiting therapy to single-agent paclitaxel[8].
Each chemotherapy regimen has a distinct side effect profile that should be carefully considered when selecting treatment. Anthracycline use is associated with an increased risk of cardiac failure[56.57]. In patients with preexisting cardiovascular risk factors, anthracycline free regimens such as docetaxel/cyclophosphamide or carboplatin/paclitaxel are therefore preferable. Taxanes are relatively contraindicated in patients with preexisting neuropathy[58,59].
Research has shown that chemotherapy significantly impacts the quality of life of older women with early breast cancer, with clinically and statistically significant declines observed at 6 to 12 months[60]. In many cases, recovery occurs by 18 to 24 months[60]. Given the risk of chemotherapy related toxicity and its impact on quality of life in older patients, an important consideration is whether a patient is likely to live long enough to recover and ultimately benefit from chemotherapy.
3.3 Anti-HER2 therapies
Trastuzumab, pertuzumab, and trastuzumab emtansine (T-DM1) are effective HER2-targeted (neo)adjuvant therapies for early HER2+ breast cancer. When indicated, anti-HER2 therapy is typically initiated in the neoadjuvant setting alongside chemotherapy. However, data on the efficacy and safety of anti-HER2 therapy in older patients remain very limited.
A review of RCTs of trastuzumab combined with chemotherapy for early breast cancer found that the addition of trastuzumab significantly improved OS (HR = 0.66; 95% CI, 0.57 to 0.77; p < 0.00001)[61]. However, most trial participants were younger (median age 49 years), healthier, and had few comorbidities[61].
The combination of trastuzumab and pertuzumab has also demonstrated efficacy. The NeoSphere trial showed that adding pertuzumab to trastuzumab–docetaxel neoadjuvant therapy for 12 weeks significantly increased the pathological complete response rate from 29.0% to 45.8%[62]. Notably, the median participant age remained around 49 to 50 years[62]. Additionally, the TRAIN-2 study found that in HER2+ breast cancer, anthracycline-free neoadjuvant chemotherapy with dual HER2 blockade offers similar efficacy with potentially less toxicity compared to anthracycline-containing regimens[63]. Unfortunately, the APHINITY trial demonstrated that in the adjuvant setting, adding pertuzumab to chemotherapy and trastuzumab did not improve overall survival[64].
In women with residual disease after neoadjuvant chemotherapy combined with anti-HER2 therapy, T-DM1, an antibody drug conjugate combining trastuzumab with the cytotoxic agent emtansine (DM1), has shown superior outcomes compared to trastuzumab alone. In the randomized KATHERINE trial, the seven-year OS rate was 89.1% for T-DM1 compared to 84.4% for trastuzumab, corresponding to an unstratified HR of 0.66 (95% CI, 0.51 to 0.87; p = 0.003), representing a 34% improvement in OS with T-DM1[65]. Nonetheless, older patients remained underrepresented, with a median age of 49 years (range 23-80 in the trastuzumab group; 24-79 in the T-DM1 group)[65].
3.3.1 Safety
Trastuzumab and pertuzumab are associated with an increased risk of cardiac toxicity, particularly in individuals aged 80 years and older[66]. Pertuzumab is commonly linked to side effects such as anorexia, vomiting, dysgeusia, and diarrhea[8]. Due to these adverse effects, its use should be reserved for high-risk, fit older women.
When chemotherapy is considered contraindicated, trastuzumab monotherapy may serve as a viable alternative, although its efficacy is lower[67]. In HR+ HER2+ breast cancer, combining trastuzumab with hormonal therapy instead of chemotherapy improves outcomes while minimizing toxicity[67].
3.4 Immunotherapy
Immune checkpoint inhibitors such as pembrolizumab have revolutionized the treatment of several cancers by blocking inhibitory proteins like programmed cell death protein 1 and programmed death-ligand 1, thereby enhancing T-cell-mediated tumor destruction. The KEYNOTE 522 trial, which evaluated pembrolizumab in combination with neoadjuvant chemotherapy (paclitaxel and carboplatin followed by doxorubicin and cyclophosphamide or epirubicin and cyclophosphamide) in stage II and III triple-negative breast cancer, demonstrated significant improvements in pathological complete response rates, event-free survival, and overall survival[68]. Only 11% of participants were aged 65 years or older, and unfortunately subgroup analysis showed that this older group did not benefit from the treatment.
3.5 Emerging therapies
Recent advances in molecular oncology have expanded the arsenal of targeted agents for breast cancer treatment. These agents were originally developed for metastatic disease, but increasing attention is being given to their potential role in the (neo)adjuvant setting for early breast cancer. Among the drug classes now extensively studied, often in combination, are selective estrogen receptor degraders (SERDs), antibody-drug conjugates, PI3K inhibitors, mTOR inhibitors, and poly (ADP-ribose) polymerase (PARP) inhibitors.
4. Strategies for Personalizing Treatment Decisions
For older breast cancer patients, a personalized, patient-centered treatment approach is essential to ensure that the benefits of therapy outweigh the associated risks while aligning with the patient’s values, goals, and priorities. This approach promotes both effective care and quality of life. However, defining what constitutes a substantial benefit or accurately assessing an individual’s specific risks remains challenging. The absolute benefit of (neo)adjuvant systemic treatment depends on the risk of breast cancer mortality and the relative risk reduction achievable with treatment. In older patients, competing risks from other-cause mortality may limit the potential benefit related to breast cancer. As illustrated in Figure 1, women with an elevated risk of other cause mortality due to the presence of comorbidities or frailty experience a reduced potential benefit from treatment.
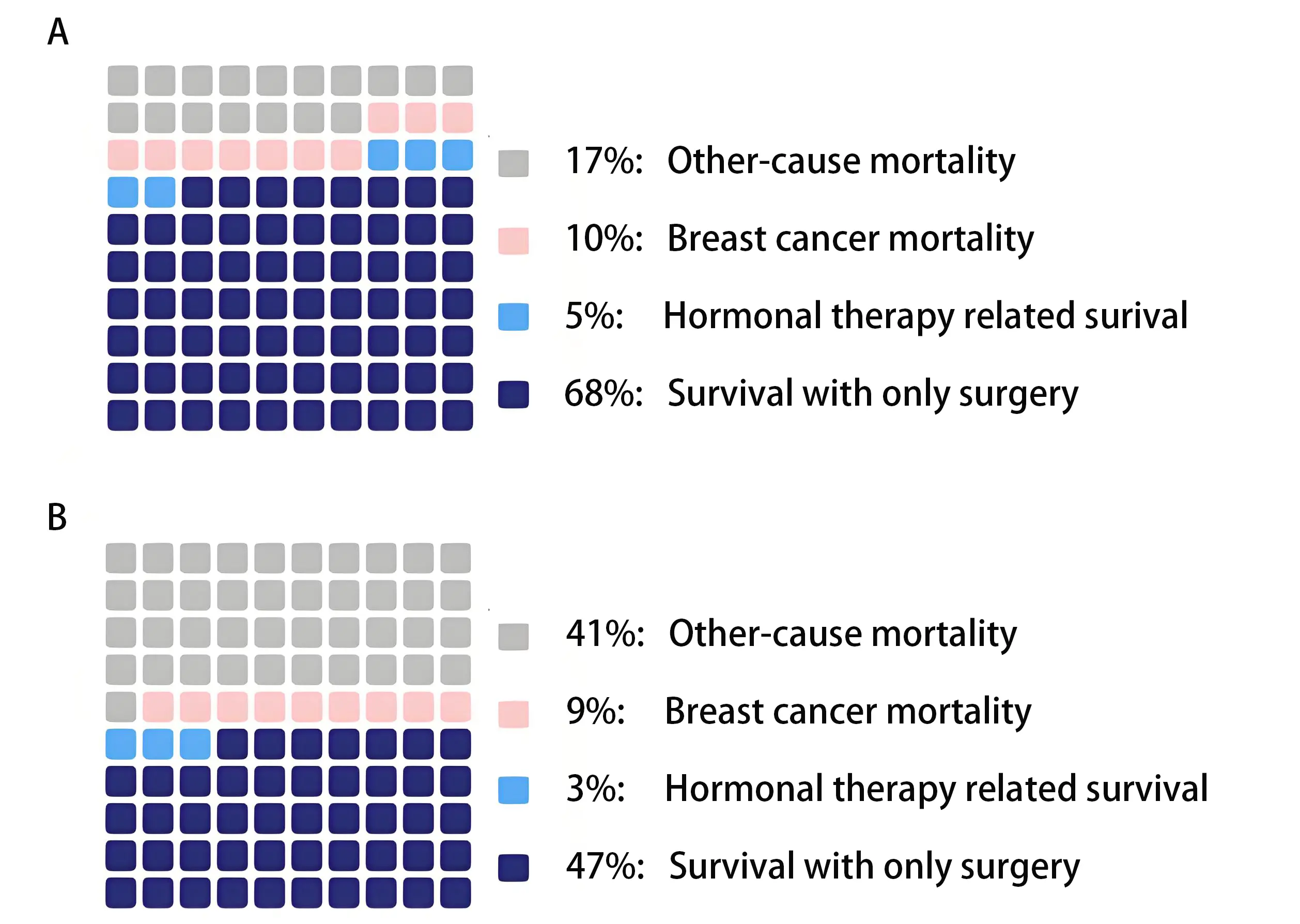
Figure 1. In older patients, competing mortality risks may reduce the potential benefit of systemic therapy. Survival estimates were generated using the PORTRET tool (version 2.7)[69]. (A) Estimated 5-year survival of a 78-year-old patient diagnosed with a 35 mm, grade 3, hormone receptor-positive, HER2-negative, Ki-67-negative breast tumor, with no lymph node involvement. The cancer was detected due to symptoms; (B) Estimated 5-year survival of a patient with identical tumor characteristics as Patient A, but with additional geriatric risk factors: six comorbidities, polypharmacy, walking difficulties, and the use of a hearing aid.
To support individualized treatment decisions, several strategies can be employed to assess risks and clarify patient preferences. These include comprehensive geriatric assessments (CGA), the use of prediction tools, and decision aids. The following sections will explore these approaches and their potential role in optimizing treatment decisions.
4.1 Comprehensive geriatric assessments
CGA plays a crucial role in tailoring adjuvant treatment strategies for older breast cancer patients by evaluating functional status, comorbidities, and psychosocial factors. CGA has been shown to predict chemotherapy-related toxicities and mortality, thereby helping clinicians balance treatment benefits and risks[70].
RCTs have demonstrated significantly improved clinical outcomes when geriatric assessment-guided management (GAM) is incorporated into care plans for cancer patients aged 65 years and older undergoing systemic therapy[71,72]. Specifically, grade 3 to 5 toxicity is reduced with GAM. One study reported that only 51% of patients in the intervention group experienced grade 3 to 5 toxicity, compared to 71% in the usual care group (RR = 0.74; 95% CI, 0.64 to 0.86; p = 0.0001)[71]. Another study found the incidence of grade 3 or higher toxicity in the intervention group was 50.5%, compared to 60.6% in the usual care group, representing a significant 10.1% reduction (95% CI, -1.5 to -18.2%; p = 0.02)[72].
Accordingly, the American Society of Clinical Oncology (ASCO) guidelines currently recommend integrating GAM for patients identified with geriatric assessment-related deficits. Furthermore, CGA should guide cancer treatment decisions and address impairments through targeted interventions, counseling, and referrals[73].
However, implementing CGA in routine clinical practice presents challenges, primarily due to its time-intensive nature and the lack of standardized tools and formats[74]. To address these issues, the practical geriatric assessment instrument (PGA) was developed. Created through an iterative process involving content experts, oncologists, and patient partners, the PGA offers practical guidance on which tools to use across various clinical settings and provides actionable, result-based advice[73,75]. While the PGA is a composite tool and has not been validated in its entirety, its individual components have been validated for use in older cancer patients. The PGA is currently endorsed by the 2023 ASCO guidelines and the Science and Education Committee of the International Society of Geriatric Oncology[73].
To further streamline CGA implementation, ASCO and ESMO guidelines propose initial screening with tools such as the G8 questionnaire to identify patients most likely to benefit from a full CGA. This approach optimizes resource allocation and ensures comprehensive assessments are applied where most needed[8,45].
4.2 Prediction tools
4.2.1 Survival prediction tools
Prediction tools provide personalized risk estimates to support evidence-based treatment planning for patients. By enabling tailored treatment plans, facilitating shared decision-making, and helping clinicians avoid both under- and overtreatment, these tools represent a valuable advancement in oncology care. In older patients, mortality from other causes may compete with breast cancer-related mortality, thereby influencing the potential benefit of treatments. Therefore, identifying patients more likely to die from causes other than breast cancer is crucial for guiding treatment decisions.
While commonly used prediction models offer important insights into treatment benefits and prognosis, they often fall short in accounting for competing mortality risks in the elderly population. One widely used, the PREDICT tool version 2.0, utilizes traditional clinicopathological variables to estimate prognosis and treatment benefits in breast cancer patients[76,77]. However, although the PREDICT tool version 2.0 performs well in the general population, its accuracy for older patients is limited due to its inability to account for competing mortality risks associated with frailty and comorbidities[78,79]. Specifically, for patients without comorbidities, breast cancer mortality and the benefit of adjuvant treatments are underestimated, whereas for patients with comorbidities, both are overestimated. PREDICT version 3.0 incorporates additional variables, such as smoking status, but remains unvalidated in older patient cohorts[77].
A validated alternative specifically developed for older patients is the PORTRET tool. By integrating clinicopathological variables along with comorbidity and geriatric factors, the PORTRET tool provides individualized estimates of both breast cancer-specific mortality and mortality from other causes. This comprehensive approach enables clinicians to assess whether a patient’s overall survival is sufficient to justify treatment aimed at reducing the risk of breast cancer recurrence. A recent study has both internally and externally validated the PORTRET tool’s predictive ability for this population[80]. The tool is expected to be available online in the coming year.
4.2.2 Toxicity prediction tool
The Cancer and Aging Research Group-Breast Cancer (CARG-BC) model, specifically designed for older women, demonstrates reasonable predictive ability for chemotherapy toxicity with an area under the curve (AUC) of 0.69, using eight clinical and geriatric variables. The CARG-BC score effectively stratifies patients aged 65 years and older with early-stage breast cancer into low-, intermediate-, and high-risk categories for grade 3 to 5 chemotherapy toxicity. It outperforms the physician-rated Karnofsky Performance Status Scale in predicting grade 3 to 5 toxicities (AUC = 0.50)[55]. However, its applicability outside the United States requires further validation.
4.3 Decision aids
Decision aids help bridge information gaps, enabling patients to make informed treatment decisions. These tools simplify complex medical information and facilitate shared decision-making[81]. For older patients, who may prioritize maintaining quality of life over aggressive treatments, decision aids can be particularly valuable. They clarify the benefits and risks of systemic therapies, making complex choices more understandable. Collaborative doctor-patient consultations guided by decision aids can reduce decisional conflict and improve treatment adherence[81].
Studies show that decision aids reduce decisional conflict without negatively impacting patients’ knowledge, anxiety, or decision regret[81,82]. Effective formats for older patients include brochures with simple language and visual aids such as charts and graphs. Web-based tools can provide detailed information but may be less accessible due to technological barriers. Tailoring decision aids to local contexts, literacy levels, and cultural preferences is essential to maximize their reach and impact[81].
Option Grids, a decision aid offering side-by-side treatment comparisons alongside answers to the most relevant and frequently asked patient questions, have proven particularly effective in enhancing patient confidence and engagement during consultations[83]. Specific examples of decision aids beneficial for older breast cancer patients include the pamphlet developed by Mara A. Schonberg et al.[84] and other decision support interventions[85].
4.4 Gene expression signatures
Gene expression signatures provide prognostic insights into recurrence risk and predictive guidance for chemotherapy response[86]. Despite their potential, gene expression tests are costly, and most signatures have been developed and validated predominantly in relatively young patient cohorts, with limited evaluation in older populations[87].
Several gene expression assays, including Oncotype DX, the HOXB13:IL17B ratio (a two-gene expression ratio comprising the homeobox gene HOXB13 and interleukin-17B receptor), MammaPrint, and the Prosigna Risk of Recurrence (ROR) score, have been analyzed in older patients[87]. No prognostic effect of the HOXB13:IL17B ratio on relapse-free survival, disease-free survival, or OS was observed[88]. Oncotype DX and the ROR score have demonstrated prognostic validity in older patients with HR+ breast cancer[87,89]. Additionally, MammaPrint has shown validity in assessing recurrence risk in older patients[90]. The predictive value of Oncotype DX appears promising particularly for intermediate-risk older patients; however, studies often included only individuals aged 65 to 75 years, limiting generalizability to the broader older population[87].
Further research is necessary to establish the predictive value of gene expression signatures in older patients before their routine clinical implementation for chemotherapy decision-making.[87].
5. Conclusion
The treatment of older patients with early invasive breast cancer requires a personalized, patient-centered approach that addresses the biological, clinical, and psychosocial complexities unique to this population. Adjuvant hormonal therapy remains the cornerstone of treatment for HR+ breast cancer. Neoadjuvant therapy should primarily be considered when tumor downstaging is desired. Chemotherapy and anti-HER2 therapies are appropriate for relatively fit older patients with high-risk subtypes, such as triple-negative and HER2-positive breast cancer. The addition of adjuvant bisphosphonates is recommended not only to maintain bone health but also to reduce the risk of breast cancer recurrence. Beyond these established modalities, emerging targeted therapies offer promising new avenues for early breast cancer treatment. However, larger studies including more older patients are essential before these novel therapies can be effectively and safely integrated into personalized treatment strategies.
The underrepresentation of older patients in clinical trials remains a major challenge, resulting in substantial evidence gaps. Increasing the inclusion of older adults in clinical trials should remain a priority.
Although this review primarily reflects evidence from high-income countries and European guidelines, it is important to recognize that clinical practices and resource availability vary widely across regions. In many low- and middle-income countries, economic constraints, limited diagnostic capabilities, and restricted access to therapeutic agents significantly affect treatment approaches. Consequently, protocols involving costly targeted therapies or advanced imaging for response evaluation may not be directly applicable. Moreover, prediction tools may be less reliable in these settings and require careful validation. To ensure that personalized, patient-centered care is both effective and feasible globally, future research should focus on generating evidence from more diverse populations. Only then can both individual complexities and broader healthcare realities be adequately addressed.
The absolute benefit of (neo)adjuvant systemic treatment depends on baseline breast cancer mortality risk and the achievable relative risk reduction. In older patients, competing mortality from other causes diminishes the potential benefit of treatment. Therefore, optimal systemic treatment decisions, particularly for older women with HR+ disease, require careful weighing of these factors.
CGA plays a vital role in identifying patient vulnerabilities and assessing frailty. Predictive models, such as the PORTRET tool and CARG-BC score, incorporate geriatric factors to estimate survival and toxicity risks. These tools assist clinicians and patients in balancing the benefits and potential harms of systemic treatment. Systemic therapies should be initiated only when both patient and physician agree that the expected survival benefit is substantial. Decision aids, such as option grids, facilitate shared decision-making and ensure treatment choices align with the patient’s values, goals, and life priorities. By integrating these components including frailty assessment, predictive tools, and shared decision-making, as illustrated in Figure 2, we move closer to truly personalized care that minimizes both undertreatment and overtreatment among older breast cancer patients.
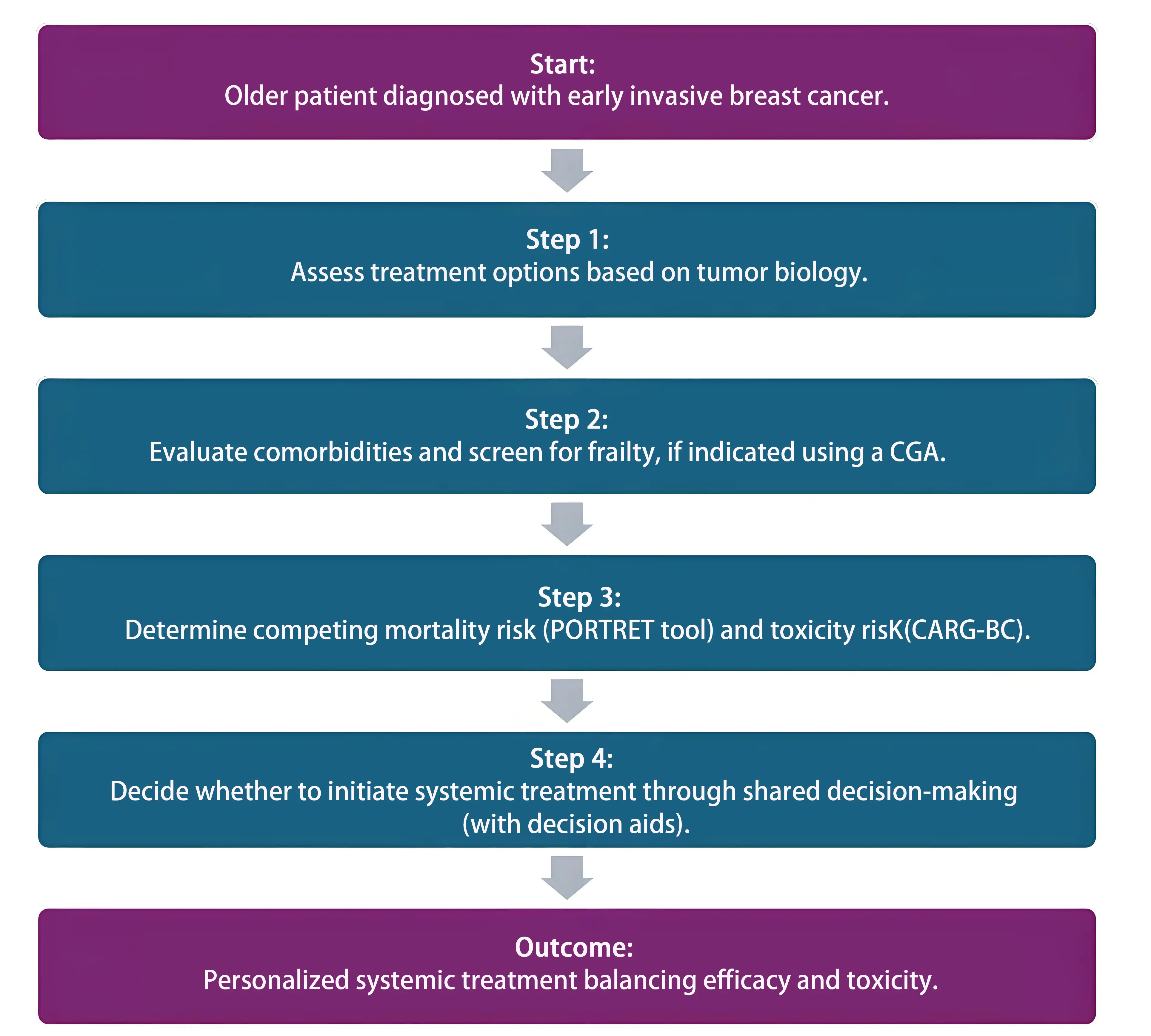
Figure 2. Personalized systemic treatment decision flowchart. This flowchart outlines a stepwise approach to personalized systemic treatment decisions in older patients, incorporating tumor biology, frailty screening, mortality risk prediction, and shared decision-making. CGA: comprehensive geriatric assessments; CARG-BC: Cancer and Aging Research Group-Breast Cancer.
Authors contribution
Wolbink JN: Contributed to the conception of the review, synthesized the collected evidence, drafted the manuscript.
van Bodegom-Vos L, Blok EJ: Substantively revised manuscript.
Portielje JEA: Contributed to the conception of the review, collected evidence, substantively revised manuscript.
Conflict of interest
Johanneke E. A. Portielje is an Editorial Board member of Ageing and Cancer Research & Treatment. The other authors declared that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Funding
This work was supported by the Dutch Cancer Foundation (KWF) under Grant 2023–15744: Implementation of the PORTRET tool in daily clinical practice.
Copyright
© The Author(s) 2025.
References
-
1. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Sauer AG, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438-451.
[DOI] -
2. Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091-1101.
[DOI] -
3. Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14.
[DOI] -
4. Glas N, Bastiaannet E, de Boer A, Siesling S, Liefers GJ, Portielje J. Improved survival of older patients with advanced breast cancer due to an increase in systemic treatments: a population-based study. Breast Cancer Res Treat. 2019;178(1):141-149.
[DOI] -
5. Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psychooncology. 2019;28(7):1367-1380.
[DOI] -
6. Ludmir EB, Mainwaring W, Lin TA, Miller AB, Jethanandani A, Espinoza AF, et al. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol. 2019;5(12):1769-1773.
[DOI] -
7. Singh H, Beaver JA, Kim G, Pazdur R. Enrollment of older adults on oncology trials: An FDA perspective. J Geriatr Oncol. May 2017;8(3):149-150.
[DOI] -
8. Biganzoli L, Battisti NML, Wildiers H, McCartney A, Colloca G, Kunkle IH, et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021;22(7):e327-e340.
[DOI] -
9. Richtlijnendatabase [Internet]. Startpagina-Borstkanker. Utrecht: Federatie Medisch Specialisten; [cited 2025 Jan]. Available from: https://richtlijnendatabase.nl/richtlijn/borstkanker/startpagina_-_borstkanker.html
-
10. Lodi M, Bousquet N, Valverde P, De la Ferrière M, Neuberger K, Jankowski S, et al. Breast cancer characteristics in elderly women: A comprehensive cohort study of 7,965 patients. Innov Pract Breast Health. 2024;1:100001.
[DOI] -
11. Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92(7):550-556.
[DOI] -
12. Lemij AA, Bastiaannet E, de Glas NA, van den Bos F, Portielje JEA, Liefers GJ, et al. Breast cancer in the older population: a global challenge-an epidemiological perspective. Ann Breast Surg. 2023;7:17.
[DOI] -
13. van der Plas-Krijgsman WG, Morgan JL, de Glas NA, de Boer AZ, Martin CL, Holmes GR, et al. Differences in treatment and survival of older patients with operable breast cancer between the United Kingdom and the Netherlands - A comparison of two national prospective longitudinal multi-centre cohort studies. Eur J Cancer. 2022;163:189-199.
[DOI] -
14. Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, et al. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013;31(25):3083-3090.
[DOI] -
15. Cheun JH, Kim HK, Moon HG, Han W, Lee HB. Locoregional recurrence patterns in patients with different molecular subtypes of breast cancer. JAMA Surgery. 2023;158(8):841-852.
-
16. Arecco L, Bruzzone M, Bas R, Kim HJ, Meglio AD, Bernstein-Molho R, et al. Impact of hormone receptor status and tumor subtypes of breast cancer in young BRCA carriers. Ann Oncol. 2024;35(9):792-804.
[DOI] -
17. Lee YJ, Yoo TK, Kim J, Chung Y, Ko BS, Kim HJ, et al. Survival outcomes of breast cancer patients with recurrence after surgery according to period and subtype. PLoS One. 2023;18(7):e0284460.
[DOI] -
18. Moo TA, Sanford R, Dang C, Morrow M. Overview of breast cancer therapy. PET Clin. 2018;13(3):339-354.
[DOI] -
19. Vasigh M, Karoobi M, Williams AD, Abreha FM, Bleicher RJ, Yazd SMM, et al. Neoadjuvant endocrine therapy compared to neoadjuvant chemotherapy in node-positive HR+, HER2- breast cancer (nodal pCR and the rate of ALND): A systematic review and meta-analysis. Breast J. 2024;2024(1):8866756. [DOI: https://doi.org/10.1155/2024/8866756]
[DOI] -
20. Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110(2):244-254.
[DOI] -
21. Chu KC, Anderson WF. Rates for breast cancer characteristics by estrogen and progesterone receptor status in the major racial/ethnic groups. Breast Cancer Res Treat. 2002;74(3):199-211.
[DOI] -
22. Clusan L, Ferrière F, Flouriot G, Pakdel F. A basic review on estrogen receptor signaling pathways in breast cancer. Int J Mol Sci. 2023;24(7):6834.
[DOI] -
23. Abdel-Razeq H, Abu Rous F, Abuhijla F, Abdel-Razeq N, Edaily S. Breast cancer in geriatric patients: Current landscape and future prospects. Clin Interv Aging. 2022;17:1445-1460.
[DOI] -
24. Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717.
[DOI] -
25. Crystal JS, Rand J, Johnson J, Kim S, Basho R, Amersi F, et al. Adjuvant endocrine therapy is associated with improved overall survival in elderly hormone receptor-positive breast cancer patients. Breast Cancer Res Treat. 2020;184(1):63-74.
[DOI] -
26. Khan AJ, Parikh RR, Neboori HJ, Goyal S, Haffty BG, Moran MS. The relative benefits of tamoxifen in older women with T1 early-stage breast cancer treated with breast-conserving surgery and radiation therapy. Breast J. 2013;19(5):490-495.
[DOI] -
27. Christiansen P, Bjerre K, Ejlertsen B, Jensen MB, Rasmussen BB, Lænkholm AV, et al. Mortality rates among early-stage hormone receptor-positive breast cancer patients: a population-based cohort study in Denmark. J Natl Cancer Inst. 2011;103(18):1363-1372.
[DOI] -
28. Alba E, Calvo L, Albanell J, De la Haba JR, Lanza AA, Chacon JI, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23(12):3069-3074.
[DOI] -
29. Oh DS, Troester MA, Usary J, Hu Z, He X, Fan C, et al. Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J Clin Oncol. 2006;24(11):1656-1664.
[DOI] -
30. Barnadas A, Gil M, Sánchez-Rovira P, Llombart A, Adrover E, Estevez L, et al. Neoadjuvant endocrine therapy for breast cancer: past, present and future. Anticancer Drugs. 2008;19(4):339-347.
[DOI] -
31. Pawloski KR, Barrio AV. Breast surgery after neoadjuvant systemic therapy. Transl Breast Cancer Res. 2024;5:13.
[DOI] -
32. van de Loo ME, Andour L, van Heesewijk AE, Oosterkamp HM, Liefers GJ, Straver ME. Neoadjuvant endocrine treatment in hormone receptor-positive breast cancer: Does it result in more breast-conserving surgery? Breast Cancer Res Treat. 2024;205(1):5-16.
[DOI] -
33. Tamirisa N, Lin H, Shen Y, Shaitelman SF, Karuturi MS, Giordano SH, et al. Impact of adjuvant endocrine therapy in older patients with comorbidities and estrogen receptor-positive, node-negative breast cancer-A National Cancer Database analysis. Cancer. 2021;127(13):2196-2203.
[DOI] -
34. McDuff SGR, Blitzblau RC. Optimizing adjuvant treatment recommendations for older women with biologically favorable breast cancer: Short-course radiation or long-course endocrine therapy? Curr Oncol. 2022;30(1):392-400.
[DOI] -
35. Crivellari D, Spazzapan S, Puglisi F, Fratino L, Scalone S, Veronesi A. Hormone therapy in elderly breast cancer patients with comorbidities. Crit Rev Oncol Hematol. 2010;73(1):92-98.
[DOI] -
36. Botteman M, Barghout V, Stephens J, Hay J, Brandman J, Aapro M. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol. 2006;17(7):1072-1082.
[DOI] -
37. Liu Y, Zhao S, Zhang Y, Onwuka JU, Zhang Q, Liu X. Bisphosphonates and breast cancer survival: a meta-analysis and trial sequential analysis of 81508 participants from 23 prospective epidemiological studies. Aging. 2021;13(15):19835-19866.
[DOI] -
38. Korde LA, Doody DR, Hsu L, Porter PL, Malone KE. Bisphosphonate use and risk of recurrence, second primary breast cancer, and breast cancer mortality in a population-based cohort of breast cancer patients. Cancer Epidemiol Biomarkers Prev. 2018;27(2):165-173.
[DOI] -
39. Lemij AA, de Glas NA, Derks MGM, Bastiaannet E, Merkus JWS, Lans TE, et al. Discontinuation of adjuvant endocrine therapy and impact on quality of life and functional status in older patients with breast cancer. Breast Cancer Res Treat. 2022;193(3):567-577.
[DOI] -
40. Baltussen JC, Derks MGM, Lemij AA, de Glas NA, Fiocco M, Linthorst-Niers EMH, et al. Association between endocrine therapy and cognitive decline in older women with early breast cancer: Findings from the prospective CLIMB study. Eur J Cancer. 2023;185:1-10.
[DOI] -
41. Rastogi P, O'Shaughnessy J, Martin M, Boyle F, Cortes J, Rugo HS, et al. Adjuvant abemaciclib plus endocrine therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative, high-risk early breast cancer: Results from a preplanned monarchE overall survival interim analysis, including 5-year efficacy outcomes. J Clin Oncol. 2024;42(9):987-993.
[DOI] -
42. Slamon DJ, Stroyakovskiy D, Yardley DA, Huang CS, Fasching PA, Crown J, et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: Primary results from the phase III NATALEE trial. J Clin Oncol. 2023;41:LBA500.
[DOI] -
43. Bagnyukova TV, Serebriiskii IG, Zhou Y, Hopper-Borge EA, Golemis EA, Astsaturov I. Chemotherapy and signaling: How can targeted therapies supercharge cytotoxic agents? Cancer Biol Ther. 2010;10(9):839-853.
[DOI] -
44. Braybrooke J, Bradley R, Gray R, Hills RK, Pan H, Peto R, et al. Lancet 2023;
[DOI] -
45. Trapani D. Adjuvant chemotherapy in older women with early breast cancer. J Clin Oncol. 2023;41(9):1652-1658.
[DOI] -
46. Brain E, Viansone AA, Bourbouloux E, Rigal O, Ferrero JM, Sylvie K, et al. Final results from a phase III randomized clinical trial of adjuvant endocrine therapy ± chemotherapy in women ≥ 70 years old with ER+ HER2- breast cancer and a high genomic grade index: The Unicancer ASTER 70s trial. J Clin Oncol. 2022;40:500.
[DOI] -
47. Schmidt M, Loibl S. Chemotherapy in older patients with early breast cancer. Breast. 2024;78:103821.
[DOI] -
48. Schmidt M, Nitz U, Reimer T, Schmatloch S, Graf H, Just M, et al. Adjuvant capecitabine versus nihil in older patients with node-positive/high-risk node-negative early breast cancer receiving ibandronate - The ICE randomized clinical trial. Eur J Cancer. 2023;194:113324.
[DOI] -
49. Elkin EB, Hurria A, Mitra N, Schrag D, Panageas KS. Adjuvant chemotherapy and survival in older women with hormone receptor-negative breast cancer: Assessing outcome in a population-based, observational cohort. J Clin Oncol. 2006;24(18):2757-2764.
[DOI] -
50. Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24(18):2750-2756.
[DOI] -
51. Williams AD, Dang CT, Sevilimedu V, Morrow M, Barrio AV. Neoadjuvant chemotherapy for breast cancer in the elderly: Are we accomplishing our treatment goals? Ann Surg Oncol. 2022;29(13):8002-8011.
[DOI] -
52. Mantilla W, Gonzalez M, Rojas S, Borras-Osorio M, Molano-Gonzalez N, Moran D, et al. Significance of pathologic response in patients with early and locally advanced breast cancer treated with neoadjuvant chemotherapy in a middle-income country. A real-world historical cohort. J Clin Oncol. 2024;(10):e2300187.
[DOI] -
53. Lichtman SM, Cirrincione CT, Hurria A, Jatoi A, Theodoulou M, Wolff AC, et al. Effect of pretreatment renal function on treatment and clinical outcomes in the adjuvant treatment of older women with breast cancer: Alliance A171201, an ancillary study of CALGB/CTSU 49907. J Clin Oncol. 2016;34(7):699-705.
[DOI] -
54. Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465.
[DOI] -
55. Magnuson A, Sedrak MS, Gross CP, TewWP , Klepin HD, Wildes TM, et al. Development and validation of a risk tool for predicting severe toxicity in older adults receiving chemotherapy for early-stage breast cancer. J Clin Oncol. 2021;39(6):608-618.
[DOI] -
56. Zhang M, Yang H, Xu C, Jin F, Zheng A. Risk factors for anthracycline-induced cardiotoxicity in breast cancer treatment: A meta-analysis. Front Oncol. 2022;12:899782.
[DOI] -
57. Volkova M, Russell R. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7(4):214-220.
[DOI] -
58. Costa R, Passos GF, Quintão NLM, Fernandes ES, Maia JRLCB, Campos MM, et al. Taxane-induced neurotoxicity: Pathophysiology and therapeutic perspectives. Br J Pharmacol. 2020;177(14):3127-3146.
[DOI] -
59. Rivera E, Cianfrocca M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother Pharmacol. 2015;75(4):659-670.
[DOI] -
60. Battisti NML, Reed MWR, Herbert E, Morgan JL, Collins KA, Ward SE, et al. Bridging the age gap in breast cancer: Impact of chemotherapy on quality of life in older women with early breast cancer. Eur J Cancer. 2021;144:269-280.
[DOI] -
61. Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2021;4:CD006243.
[DOI] -
62. Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25-32.
[DOI] -
63. Ramshorst MS, van der, van Werkhoven, Mandjes IA, Kemper I, Dezentjé VO, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1630-1640.
[DOI] -
64. Loibl S, Jassem J, Sonnenblick A, Parlier D, Winer E, Bergh J, et al. Adjuvant pertuzumab and trastuzumab in early human epidermal growth factor receptor 2-positive breast cancer in the APHINITY trial: Third interim overall survival analysis with efficacy update. J Clin Oncol. 2024;42(31):3643-3651.
[DOI] -
65. Geyer CE, Untch M, Huang CS, Mano MS, Mamounas EP, Wolmark N, et al. Survival with trastuzumab emtansine in residual HER2-positive breast cancer. N Engl J Med. 2025;392(3):249-257.
[DOI] -
66. Denegri A, Moccetti T, Moccetti M, Spallarossa P, Brunelli C, Ameri P. Cardiac toxicity of trastuzumab in elderly patients with breast cancer. J Geriatr Cardiol. 2016;13(4):355-363.
[DOI] -
67. Pondé N, Wildiers H, Awada A, de Azambuja E, Deliens C, Lago LD. Targeted therapy for breast cancer in older patients. J Geriatr Oncol. 2020;11(3):380-388.
[DOI] -
68. Schmid P, Cortes J, Dent R, McArthur H, Pusztai L, Kümmel S, et al. Overall survival with pembrolizumab in early-stage triple-negative breast cancer. N Engl J Med. 2024;391(21):1981-1991.
[DOI] -
69. van der Plas-Krijgsman DG, Putter H, Steyerberg EW, Bastiaannet E, Stiggelbout AM, Mooijaart SP, et al. PORTRET tool (version 2.7) [software]. Evidencio [cited 2025 Jul 3]. Available from: https://evidencio.com/models/show/2917
-
70. Okonji DO, Sinha R, Phillips I, Fatz D, Ring A. Comprehensive geriatric assessment in 326 older women with early breast cancer. Br J Cancer. 2017;117(7):925-931.
[DOI] -
71. Mohile SG, Mohamed MR, Xu H, Culakova E, Loh KP, Magnuson A, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398(10314):1894-1904.
[DOI] -
72. Li D, Sun CL, Kim H, Soto-Perez-de-Celis E, Chung V, Koczywas M, et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: A randomized clinical trial. JAMA Oncol. 2021;7(11):e214158.
[DOI] -
73. Dale W, Klepin HD, Williams GR, Alibhai SMH, Bergerot C, Brintzenhofeszoc K, et al. Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO guideline update. J Clin Oncol. 2023;41(26):4293-4312.
[DOI] -
74. Puts MT, Hardt J, Monette J, Girre V, Springall E, Alibhai SM. Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst. 2012;104(15):1133-1163.
[DOI] -
75. Mohile SG, Velarde C, Hurria A, Magnuson A, Lowenstein L, Pandya C, et al. Geriatric assessment-guided care processes for older adults: A delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130.
[DOI] -
76. Wishart GC, Azzato EM, Greenberg DC, Rashbass J, Kearins O, Lawrence G, et al. PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res. 2010;12(1):R1.
[DOI] -
77. Grootes I, Wishart GC, Pharoah PDP. An updated PREDICT breast cancer prognostic model including the benefits and harms of radiotherapy. NPJ Breast Cancer. 22024;10(1):6.
[DOI] -
78. van der Plas-Krijgsman WG, de Boer AZ, de Jong P, Bastiaannet E, van den Bos F, Mooijaart SP, et al. Predicting disease-related and patient-reported outcomes in older patients with breast cancer - a systematic review. J Geriatr Oncol. 2021;12(5):696-704.
[DOI] -
79. de Glas NA, Bastiaannet E, Engels CC, de Craen AJM, Putter H, van de Velde CJH, et al. Validity of the online PREDICT tool in older patients with breast cancer: a population-based study. Br J Cancer. 2016;114(4):395-400.
[DOI] -
80. Plas-Krijgsman WG, Giardiello D, Putter H, Steyerberg EW, Bastiaannet E, Stiggelbout AM, et al. Development and validation of the PORTRET tool to predict recurrence, overall survival, and other-cause mortality in older patients with breast cancer in the Netherlands: a population-based study. Lancet Healthy Longev. 2021;2(11):e704-e711.
[DOI] -
81. Gao JP, Jin YH, Yu SF, Wu WF, Han SF. Evaluate the effectiveness of breast cancer decision aids: A systematic review and meta-analysis of randomize clinical trails. Nurs Open. 2021;8(5):2091-2104.
[DOI] -
82. Maes-Carballo M, Martin-Diaz M, Garcia-Garcia M, Reinoso-Hermida A, Mignini L, Teixeira-Arcaya RP, et al. Decision aids for decision making about locally advance breast cancer: A systematic review. Cancer Invest. 2023:41(3):292-304;
[DOI] -
83. Elwyn G, Lloyd A, Joseph-Williams N, Cording E, Thomson R, Durand MA, et al. Option Grids: shared decision making made easier. Patient Educ Couns. 2013;90(2):207-212.
[DOI] -
84. Schonberg MA, Freedman RA, Recht AR, Jacobson AR, Aliberti GM, Karamourtopoulos M, et al. Developing a patient decision aid for women aged 70 and older with early stage, estrogen receptor positive, HER2 negative, breast cancer. J Geriatr Oncol. 2019;10(6):980-986.
[DOI] -
85. Wyld L, Reed MWR, Collins K, Burton M, Lifford K, Edwards A, et al. Bridging the age gap in breast cancer: cluster randomized trial of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices. Br J Surg. 2021;108(5):499-510.
[DOI] -
86. Andre F, Ismaila N, Allison KH, Barlow WE, Collyar DE, Damodaran S, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022;40(16):1816-1837.
[DOI] -
87. Lemij AA, Baltussen JC, de Glas NA, Kroep JR, Derks MGM, Liefers GJ, et al. Gene expression signatures in older patients with breast cancer: A systematic review. Crit Rev Oncol Hematol. 2023;181:103884.
[DOI] -
88. Goetz MP, Suman VJ, Ingle JN, Nibbe AM, Visscher DW, Reynolds CA, et al. A two-gene expression ratio of homeobox 13 and interleukin-17B receptor for prediction of recurrence and survival in women receiving adjuvant tamoxifen. Clin Cancer Res. 2006;12(7):2080-2087.
[DOI] -
89. Sestak I, Dowsett M, Ferree S, Baehner FL, Cuzick J. Retrospective analysis of molecular scores for the prediction of distant recurrence according to baseline risk factors. Breast Cancer Res Treat. 2016;159(1):71-78.
[DOI] -
90. Noordhoek I, Bastiaannet E, de Glas NA, Scheepens J, Esserman LJ, Wesseling J, et al. Validation of the 70-gene signature test (MammaPrint) to identify patients with breast cancer aged ≥ 70 years with ultralow risk of distant recurrence: A population-based cohort study. J Geriatr Oncol. 2022;13(8):1172-1177.
[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




