Abstract
Radiation therapy (RT) is a cornerstone of cancer management, required in approximately half of all cancer cases, and is particularly relevant for older adults, who constitute the majority of oncology patients. Despite its localized nature and generally favorable toxicity profile, RT remains underutilized in this population, often due to age-related biases, comorbidities, or the limited integration of geriatric assessment into treatment planning. This review examines the evolving role of RT as an age-inclusive modality, highlighting innovations such as intensity-modulated and stereotactic techniques that enable more conformal, less toxic, and increasingly personalized regimens for older adults. Special attention is given to the challenges of frailty, cognitive impairment, and movement disorders, which may complicate treatment delivery and necessitate tailored adaptations. The role of comprehensive geriatric assessment and frailty screening tools is critically appraised, with emphasis on their predictive value in identifying treatment-limiting vulnerabilities and supporting shared decision-making. The review underscores the need to shift from age-based to function-based treatment paradigms, advocating for greater inclusion of older adults in clinical trials and for a multidisciplinary approach that aligns oncologic goals with patient priorities. When appropriately tailored, RT provides a safe, effective, and goal-concordant treatment option for older adults, and its optimized integration into geriatric oncology care is essential to meet the needs of an aging global population.
Keywords
1. Introduction
Cancer is strongly associated with aging, with approximately 60% of new diagnoses and 70% of cancer-related deaths occurring in individuals aged 65 years and older[1]. As global life expectancy continues to rise, the proportion of older adults living with cancer is projected to increase substantially, creating unique challenges for oncology care delivery[2,3].
Radiation therapy (RT) remains a cornerstone of cancer treatment, employed in about half of all cancer cases either with curative intent or for palliation[4]. For older adults, RT offers a non-invasive option that may be preferable to surgery or systemic therapies, particularly in the presence of comorbidities and frailty. As a localized treatment, RT is generally well tolerated by older patients and can often be completed within a relatively short timeframe[5,6]. Thus, chronological age alone should not be considered a contraindication to RT. Advances in technology have markedly improved the precision and safety of RT, enabling more accurate tumor targeting while minimizing damage to surrounding healthy tissues. These innovations are particularly beneficial for older adults, who often experience age-related physiological decline across multiple organ systems and varying degrees of frailty.
Nevertheless, RT remains underutilized in older adults. Multiple studies have documented significant age-related disparities in RT use, which are often not fully explained by comorbidities or patient preferences[7,8]. Additional factors such as lower socioeconomic status and greater distance from treatment centres further exacerbate these disparities[9]. Another persistent challenge in geriatric radiation oncology is the limited applicability of clinical trials to older populations. Despite the high incidence of cancer in this age group, there remains a substantial age gap between trial participants and the real-world patients[10,11].
In recent decades, advances in RT have markedly improved dose conformity, reduced toxicity, and enabled more individualized treatment approaches. This review highlights key modern RT techniques and their integration into geriatric oncology, while also examining the role of geriatric assessment in shifting from age-based to function-based treatment paradigms that align with patient preferences.
2. Methodology—Literature Selection
This review is based on a narrative literature survey, informed by the authors’ clinical and academic expertise in both radiation oncology and geriatric medicine. Key peer-reviewed publications, clinical guidelines, and seminal trials were selected to illustrate the evolving role of modern radiotherapy techniques in the care of older adults with cancer.
Relevant sources were identified through targeted searches of PubMed, Google Scholar, and the reference lists of recent reviews, supplemented by the authors’ knowledge of influential studies in the field. Preference was given to recent publications (2015-2025) and studies specifically reporting outcomes in older or frail populations, although earlier landmark trials were included where historically important.
The aim of this approach is to provide a concise synthesis of clinically relevant advances and their implications for geriatric oncology practice, rather than an exhaustive systematic review.
3. Contemporary RT Techniques and Their Applicability in Older Adults
Historically, three-dimensional conformal radiation therapy represented a significant advancement over two-dimensional techniques and remains in use in certain clinical settings. However, it has largely been replaced in many centers by more advanced modalities such as intensity-modulated radiation therapy (IMRT). IMRT modulates beam intensity to conform to the tumor shape, thereby reducing exposure to surrounding organs and lowering toxicity, which is particularly beneficial for frail older adults[12]. By sparing normal tissue, IMRT minimizes side effects such as fatigue, mucositis, and gastrointestinal toxicity, helping to preserve functional independence[13]. Moreover, improved tolerability increases the likelihood that older patients will complete treatment without interruptions, an important radiobiological factor in optimizing local control and overall survival[14].
Volumetric modulated arc therapy (VMAT), now widely adopted, provides a similar level of modulation while delivering radiation in a continuous arc around the patient. This efficiency shortens treatment sessions, an important advantage for older adults who may experience pain, fatigue, or cognitive impairment. Longer treatment times can increase the risk of discomfort, agitation, and involuntary movement, potentially compromising treatment accuracy and compliance. VMAT also offers strategic benefits in patients with prostate cancer and bilateral hip prostheses, as it can minimize radiation dose to prosthetic implants[15].
Stereotactic ablative body radiotherapy (SABR), also known as stereotactic body radiotherapy (SBRT), is a highly precise technique that delivers very high doses of radiation in one to five fractions to tightly targeted areas, typically with smaller margins than conventional RT. SABR has transformed the management of early-stage non-small cell lung cancer (NSCLC), offering excellent local control with acceptable toxicity in older patients who are ineligible for surgery[16].
More recently, proton beam therapy has emerged as an important development, using positively charged particles with a Bragg peak distribution to achieve maximal dose deposition at a defined depth with minimal exit dose. This characteristic makes it especially advantageous for tumors located near critical structures such as the skull base or central nervous system, with substantially reduced toxicity. However, widespread implementation remains limited due to high costs and restricted availability, and the modality is often prioritized for pediatric patients.
4. Optimizing Imaging and Immobilization Protocols for Older Patients
During RT, the position of the tumor and surrounding normal tissues may shift, either between sessions or even within a single fraction, due to factors such as respiration or bladder filling. Consequently, treatment plans based on the initial planning CT may not always accurately reflect daily internal anatomy. Alongside modern RT delivery techniques, advances in imaging and immobilization have enabled treatments that are safer and more precise than ever before.
Image-guided radiotherapy (IGRT) incorporates frequent imaging during treatment, allowing real-time adjustments for patient or tumor motion. For older adults, IGRT improves safety by reducing unnecessary exposure of healthy tissues and minimizing side effects. The enhanced accuracy of IGRT has facilitated the development of adaptive radiotherapy (ART), an advanced approach that modifies the treatment plan during the course of therapy to account for anatomical or tumor changes. Such changes may include tumor shrinkage or growth, weight fluctuations, or organ motion (e.g., bladder filling, breathing). This adaptability is particularly relevant for older patients, who are more likely to experience physiological variability due to weight loss, sarcopenia, and comorbidities. Artificial intelligence (AI) is increasingly integrated into ART workflows, using automated image recognition and decision-support systems to accelerate re-planning[17]. For older adults, AI-assisted ART can shorten on-couch adaptation times, reduce the physical and cognitive strain of prolonged immobilization, and ensure that daily anatomical variations are addressed without adding substantial treatment burden. Thus, AI-enabled ART holds considerable promise for delivering highly personalized, tolerance-based treatments in frail populations.
Surface-guided radiotherapy (SGRT) employs three-dimensional surface imaging to continuously monitor patient positioning in real time. For older patients, this approach eliminates the need for permanent tattoos or skin markings, reduces treatment-related distress, and enhances comfort. SGRT has demonstrated particular value in settings requiring high positional accuracy, such as deep inspiration breath-hold for left-sided breast cancer, where it minimizes cardiac dose, an especially important consideration for older patients with preexisting cardiovascular disease. Furthermore, the streamlined setup process offered by SGRT can shorten treatment times and reduce physical burden, benefiting patients with limited mobility, fatigue, or cognitive impairment.
Effective immobilization is essential in RT to ensure reproducible positioning and accurate dose delivery. However, this step can be challenging for older patients, who may experience discomfort due to musculoskeletal conditions such as arthritis or who may have cognitive impairments, including dementia, that contribute to disorientation and reduced compliance in the treatment environment. Open-face masks (OFMs) have emerged as a patient-centered alternative to conventional closed-face thermoplastic masks. OFMs significantly reduce anxiety and claustrophobia while maintaining positional accuracy, thereby improving patient comfort and cooperation, particularly among older adults who may struggle with immobilization[18,19].
5. RT Treatment Regimens: Hypofractionation
In addition to modern delivery techniques, fractionation schedules have also evolved in line with evidence-based practice, with many shifting toward hypofractionation. This approach is particularly convenient for older patients, as it reduces the number of treatment visits while maintaining comparable efficacy, thereby alleviating the burden of hospital travel and associated fatigue. Hypofractionation involves administering larger doses of radiation per fraction over fewer sessions compared with conventional schedules. Hypofractionated regimens are now the standard of care for several cancer sites, including early-stage breast cancer, with the FAST-Forward trial demonstrating the effectiveness of a one-week schedule (26 Gy in five fractions)[20]. In localized prostate cancer, moderate hypofractionation is an established, evidence-based option that achieves outcomes comparable to conventional fractionation, while also shortening treatment duration[21]. Ultra-hypofractionation is also emerging as a promising alternative in this setting, particularly for patients with low- or intermediate-risk disease[22]. For medically inoperable early-stage NSCLCs, SBRT, which is an ultra-hypofractionated approach, has become the standard of care, achieving local control rates of 85-95% and two- to three-year overall survival rates of 65-75%[23,24].
Short-course radiotherapy (SCRT) is another effective and well-tolerated option for older adults with rectal cancer, particularly those aged 75 years and above or with significant comorbidities. SCRT provides oncologic outcomes comparable to chemoradiation but with reduced toxicity and better preservation of autonomy[25,26]. Typically delivered as 5 Gy over five consecutive days, SCRT can be used either as neoadjuvant therapy before surgery or as a definitive treatment in patients unfit for surgery. Recent studies suggest that SCRT followed by delayed surgery may be especially advantageous for this population, striking a balance between efficacy and safety while minimizing treatment-related morbidity[25,27]. Given that older patients are often more vulnerable to the adverse effects of intensive chemoradiotherapy, SCRT offers a pragmatic alternative that maintains quality of life without compromising disease control.
6. Optimizing Care for Patients with Dementia and Parkinson’s Disease
Older adults with comorbid dementia and cancer often face disparities in cancer care. Studies have shown that individuals with both conditions are less likely to undergo cancer screening, receive staging information, or access curative treatments such as surgery, chemotherapy, or radiotherapy, compared with those without dementia[28]. This population also demonstrates higher mortality rates and greater health service utilization[29]. Recent evidence indicates that approximately 7.5% of individuals aged 75 years and older with cancer have a documented dementia diagnosis[30]. These figures suggest that a growing proportion of older adults undergoing RT are living with dementia, underscoring the need for specialized approaches within oncology services that balance treatment efficacy with quality-of-life considerations.
RT is often regarded as a preferable option over surgery or chemotherapy for these patients, as it may offer a more favorable balance of risks and benefits. However, these individuals frequently present with complex care needs, including higher rates of comorbidities and an increased likelihood of residing in long-term care facilities compared with cancer patients without dementia[31]. Decision-making regarding RT can be particularly challenging: patients may struggle to understand, retain, or consent to information about their treatment, and may find repeated hospital visits and side effect management especially burdensome. Family members and caregivers play a pivotal role in supporting patients through treatment, and their involvement is often essential for effective shared decision-making. Caregivers commonly act as advocates, interpreters of patient values, and coordinators of treatment logistics. For cognitively impaired patients, caregiver involvement helps ensure that treatment choices remain consistent with long-standing preferences and quality-of-life priorities. At the same time, gaps in caregiver availability or capacity may place additional responsibilities on oncology staff, who may lack specialized training in dementia care. Structured caregiver engagement, including family meetings, involvement in the consent process, and the provision of tailored educational resources, can enhance decision quality and improve the overall treatment experience.
Furthermore, dementia is not always adequately documented in medical records, which complicates the identification of patients requiring tailored support. To address these challenges, there is increasing emphasis on person-centered care, improved communication, environmental adaptations within RT departments, and enhanced training for healthcare professionals to better support this vulnerable population[31]. Initiatives such as OFMs can reduce patient distress, while additional strategies have been proposed to support patients during treatment[32]. These person-centered care recommendations include clear communication, environmental adaptations (e.g., clear signage, minimized noise, and reduced visual clutter), and active involvement of caregivers[33]. Staff training in dementia care, implementation of dementia-friendly environments, and flexible scheduling further address the complex needs of these patients[34]. Integrating these approaches into radiation oncology services is essential to ensure safe, effective, and compassionate care for older adults with dementia undergoing RT. An illustrative case below demonstrates how Comprehensive Geriatric Assessment (CGA) can influence treatment decisions for an older adult receiving RT.
Patients with movement disorders, such as Parkinson’s disease, also present unique challenges during RT due to motor symptoms including tremor and rigidity. Customized immobilization devices and shorter treatment sessions can help mitigate movement during therapy. Close coordination with neurology or geriatric specialist teams is essential to optimize antiparkinsonian medications and overall management. A multidisciplinary, individualized approach is recommended to ensure both safety and efficacy for patients with these conditions.
7. Geriatric Assessment as a Tool for Personalized RT in Older Adults
Multidisciplinary collaboration and shared decision-making are essential to ensure that older adults receive equitable, evidence-based, and compassionate cancer care. Close collaboration with geriatricians is particularly valuable in the oncology setting, although it is often limited by institutional resources[35]. Management of older cancer patients undergoing RT is complicated by heterogeneity in physiological reserve, comorbidities, cognitive function, and psychosocial factors, all of which influence treatment tolerance and outcomes. International societies, including the International Society of Geriatric Oncology and the American Society of Clinical Oncology, endorse CGA for all older adults undergoing cancer treatment, including RT, to support individualized treatment decisions and optimize outcomes[36,37].
CGA is a multidimensional diagnostic process that evaluates medical, functional, psychological, and social domains (Table 1). It is an essential tool for addressing age-related complexities, identifying frailty, and tailoring oncologic care accordingly. A growing body of level I evidence supports integrating CGA into oncology to guide personalized care plans; however, most studies have been conducted in medical oncology[38-42]. CGA evaluates the patient’s functional status, cognitive capacity, social support, and personal preferences[43]. It identifies vulnerabilities frequently overlooked in standard oncology assessments, including comorbidities, polypharmacy, cognitive impairment, nutritional deficits, functional dependency, and psychological distress[36,44]. These factors are independently associated with increased risk of treatment-related toxicities, hospitalization, treatment interruptions, and reduced quality of life[38,39,41,42].
| CGA Domain | Rationale | Sample Assessments/Tools |
| Functional Status | Evaluates the ability to perform basic self-care (ADLs) and complex daily tasks (IADLs). | ADL, IADL |
| Comorbidity | Assesses the presence and severity of additional chronic diseases or medical conditions. | CCI, CIRS-G |
| Cognition | Screens for memory, confusion, and cognitive impairment. | MoCA, MMSE, Mini-COG |
| Psychological | Identifies symptoms of depression, anxiety, or other mental health concerns. | GDS, HADS |
| Nutrition | Examines risk factors for malnutrition, weight loss, or poor dietary intake. | MNA, BMI and weight loss |
| Social Support | Reviews the availability and adequacy of help from family, friends, or community resources. | MOS-SSS, focused questions on social support |
| Polypharmacy | Looks at the number and appropriateness of medications, as well as potential drug interactions. | Medication review, number of medications, STOPP-START, Beers criteria |
| Mobility/Falls | Assesses walking ability, balance, and risk or history of falls. | TUG test, SPPB, falls history (previous 6 months) |
CGA: comprehensive geriatric assessment; ADL: activities of daily living; IADL: instrumental activities of daily living; CCI: charlson comorbidity index; CIRS-G: cumulative illness rating scale for geriatrics; MoCA: montreal cognitive assessment; MMSE: mini-mental state examination; GDS: geriatric depression scale; HADS: hospital anxiety and depression scale; MNA: mini nutritional assessment; BMI: body mass index; MOS-SSS: medical outcomes study social support survey; STOPP-START: screening tool of older person’s prescriptions- screening tool to alert to right treatment; TUG: timed up and go; SPPB: short physical performance battery.
CGA informs personalized treatment strategies by identifying patients who may benefit from treatment modification or intensified supportive care. Frailty-informed care has been shown to improve health-related quality of life, reduce treatment-related toxicity, and enhance adherence to curative-intent therapies[45,46]. It also helps prevent inappropriate overtreatment or undertreatment, ensuring that fit older patients are not denied potentially beneficial therapies based solely on age, while frail patients are spared aggressive interventions that could substantially compromise quality of life. In clinical practice, CGA findings can guide dose de-escalation, implementation of shorter (hypofractionated) RT schedules, enhanced symptom management, and prehabilitation interventions such as nutritional support and physiotherapy. These adjustments (Figure 1) help reduce toxicity, improve adherence, and maintain quality of life without compromising oncologic efficacy.
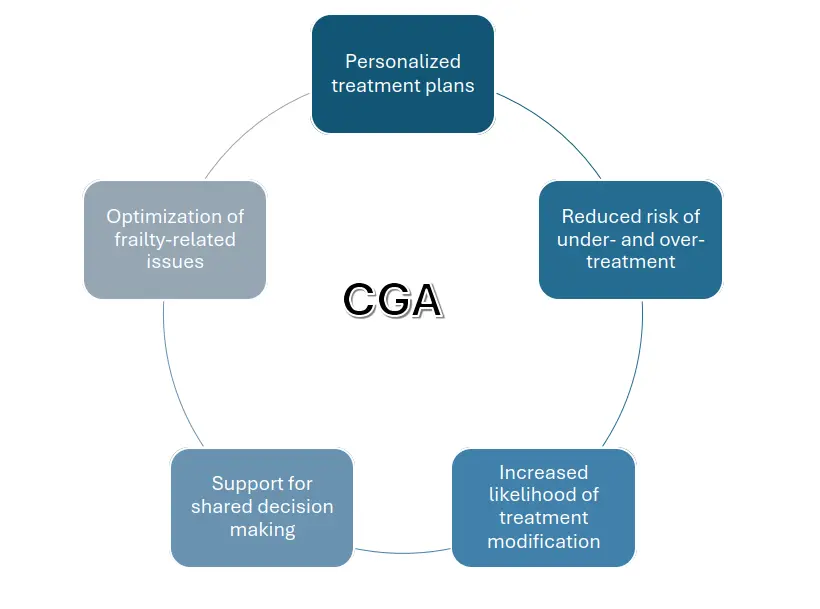
Figure 1. Impact of CGA on RT treatment decisions in older adults. CGA: comprehensive geriatric assessment; RT: radiation therapy.
Education and training of radiation oncologists in geriatric medicine principles remain critical to increase uptake and maximize the impact of CGA on patient-centered outcomes[47]. Digital health technologies and workflow integration strategies are also being explored to streamline CGA delivery in busy RT clinics[48].Despite these clear benefits, routine implementation of CGA in RT is challenged by time constraints, limited geriatric expertise within oncology teams, and resource limitations[49]. To address these barriers, brief frailty screening tools such as G8 or Vulnerable Elders Survey-13 (VES-13) are increasingly employed to identify patients who warrant full CGA, thereby optimizing resource allocation[50].
8. Frailty Screening Tools in RT
Multiple screening instruments have been evaluated in oncology and RT settings. The G8 screening tool, developed specifically for oncology, comprises eight items with a total score ranging from 0 to 17, with particular emphasis on the patient’s nutritional status. A score of ≤ 14 indicates vulnerability and warrants referral for a CGA. Validated in the ONCODAGE study, the G8 has demonstrated high sensitivity (ranging from 65% to 92%) and acceptable specificity, while requiring only 4 to 5 minutes to administer[51]. A systematic review encompassing 46 studies further supported the clinical utility of the G8, highlighting its predictive value for survival outcomes and treatment-related complications in older adults with cancer[52]. Additionally, a self-administered version of the G8 has shown good concordance with the original, suggesting potential for broader implementation in clinical practice[53].Other screening tools, including the VES-13 and the Groningen Frailty Indicator, have also been widely applied in clinical settings.
The use of frailty screening tools facilitates the identification of patients who may benefit from a full CGA. Integrating frailty screening with CGA further enhances multidisciplinary collaboration, enabling oncology teams to address polypharmacy, cognitive impairment, and social support needs, which are factors that significantly influence treatment adherence and survivorship care. In clinical practice, this approach aligns with the principles of precision medicine, optimizing therapeutic benefit while minimizing harm for vulnerable older adults.
9. Shared Decision Making and Patient Wishes
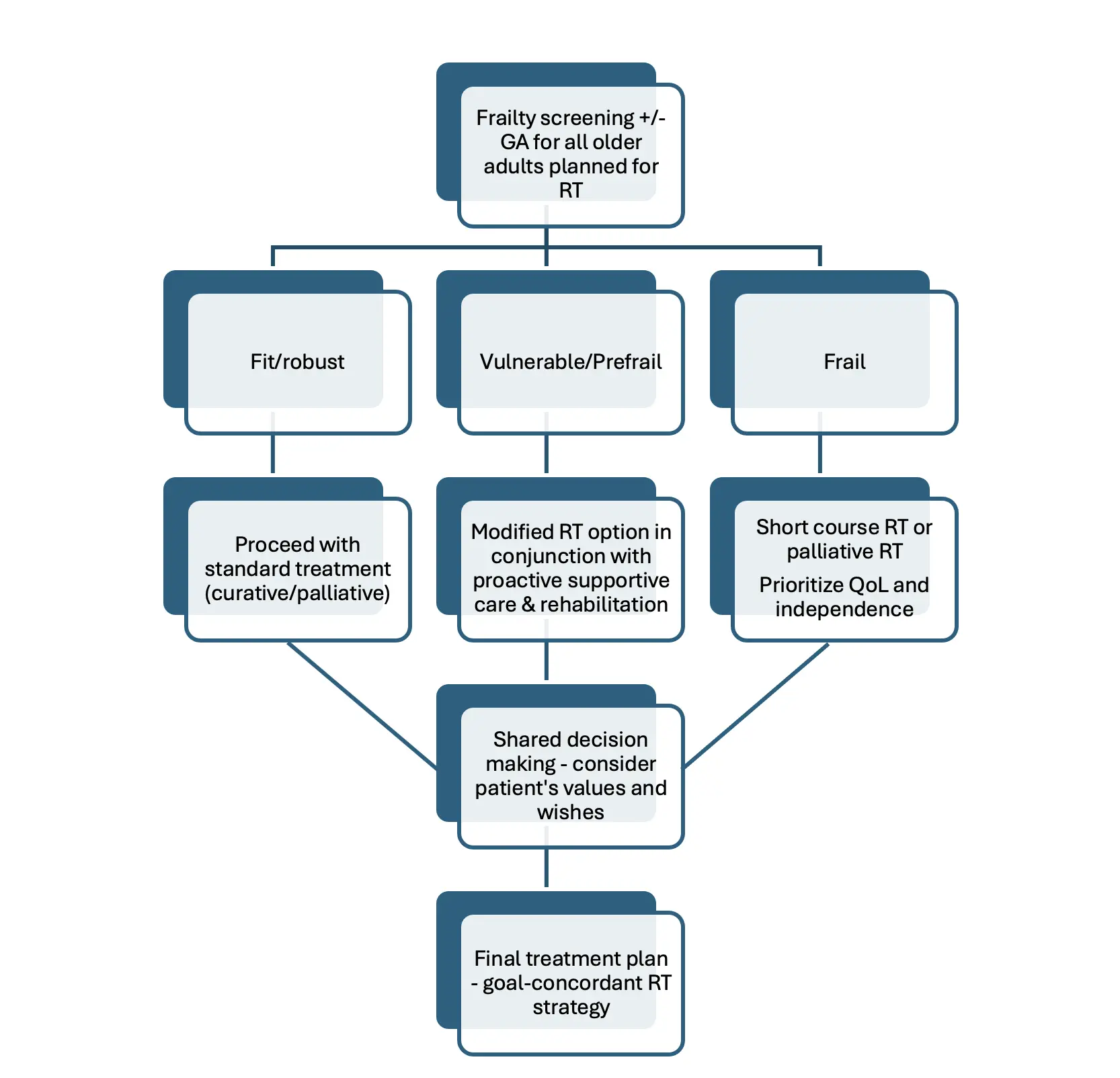
Shared decision making (SDM) is increasingly recognized as a cornerstone of cancer care for older adults, particularly in the context of RT, where treatment decisions must balance clinical evidence with the unique values and preferences of each patient[54,55]. SDM involves a collaborative process in which clinicians and patients jointly discuss treatment options, considering both potential benefits and harms in light of the patient’s health status, comorbidities, and personal priorities[55]. For older adults, these discussions are especially important, as they may prioritize quality of life over longevity or have specific fears and goals that influence their willingness to undergo certain treatments[54]. Incorporating patient wishes ensures that care plans are not only evidence-based but also aligned with what matters most to the individual, fostering greater satisfaction, trust, and adherence to treatment[55]. Despite strong patient preference for SDM in RT, studies reveal a persistent gap between the desire for involvement and actual experiences, highlighting the need for improved implementation strategies and decision aids tailored to the older population. Ultimately, respecting patient autonomy and systematically integrating their wishes into RT decisions is essential for delivering truly person-centered care in older adults (Figure 2).
10. Clinical Application: Demonstrating CGA Integration
The following illustrative case is fictional and presented for educational purposes to demonstrate how CGA findings can influence RT decision-making in older adults.
Case Study: Dementia in an Older Adult with Locally Advanced Rectal Cancer Undergoing RT
10.1 Case Presentation
An 82-year-old male with a history of mild-to-moderate Alzheimer’s disease (MMSE score: 19/30), hypertension, and osteoarthritis presented with T3N1M0 rectal adenocarcinoma. He lived with his family, required assistance with instrumental activities of daily living (IADLs), and had a Clinical Frailty Scale score of 5, indicating mild frailty. With support from his daughter, the patient expressed a strong preference to prioritize independence and minimize treatment burden rather than pursue aggressive care.
10.2 Initial Treatment Plan
The oncology team initially proposed neoadjuvant chemoradiotherapy followed by surgical resection. However, concerns were raised regarding the patient’s cognitive status, capacity to adhere to complex treatment regimens, and potential risk of treatment-related toxicity (Table 2).
| Domain | Findings | Clinical Implications |
| Cognition | MMSE 19/30; impaired executive function and short-term memory | Reduced capacity to manage complex regimens; higher risk of delirium and non-adherence |
| Nutrition | MNA score 17 → at risk of malnutrition | Nutritional vulnerability; dietician support advised |
| Physical Performance | Gait speed 0.7 m/s, SPPB 6/12 | Limited reserve; increased risk of functional decline |
| Frailty | CFS 5 (mildly frail) | Predicts higher risk from surgery and prolonged recovery |
| Psychosocial | Strong family support; no depressive symptoms | Positive caregiver involvement; buffer against treatment stress |
| Comorbidities | Controlled hypertension; no major cardiac disease | Not a limiting factor |
10.3 Treatment Modification Based on CGA
Guided by the findings of the comprehensive geriatric assessment, the multidisciplinary team recommended RT alone. After consultation with the patient, the team opted for SCRT alone (25 Gy in 5 fractions), omitting both chemotherapy and surgery. This approach aimed to minimize treatment-related toxicity while maintaining local tumor control.
10.4 Outcomes
The patient completed SCRT with only grade 1 gastrointestinal toxicity. Nutritional counseling and physiotherapy were provided to support resilience.
At 12-month follow-up:
• Oncologic: MRI demonstrated stable disease without evidence of progression.
• Functional: The patient maintained baseline independence in basic ADLs, with stable dependence in IADL.
• Cognitive: The patient continued on a gradual Alzheimer’s trajectory, with no acute cognitive decline attributable to RT.
• Quality of life: The family reported satisfaction with the balance of treatment, noting that the patient remained engaged in daily routines.
11. Future Directions
Shorter RT regimens using higher doses per fraction (hypofractionation) are now supported by robust evidence for several cancer sites. Ultra-hypofractionated schedules, which can sometimes be completed within a single week, are emerging as effective and well-tolerated options for older adults, reducing the burden of frequent hospital visits and minimizing exposure to healthcare settings. In the future, patients may be able to select and tailor RT regimens that best align with their individual needs, drawing from multiple available treatment options and guided by evidence-based practice. Incorporating geriatric assessment into radiotherapy planning enables clinicians to adapt treatments according to physiological age, comorbidities, and patient preferences. This approach helps avoid overtreatment and identifies regimens that achieve the optimal balance of efficacy and tolerability. Looking ahead, further integration of artificial intelligence into adaptive radiotherapy could facilitate highly personalized, daily-adaptive treatments in routine care. For older adults, particularly those with limited mobility or cognitive challenges, this could translate into shorter sessions and improved treatment tolerability.
Critical appraisal of the evidence base indicates that, although modern RT techniques such as IMRT, VMAT, SBRT, and hypofractionation are supported by robust data in the general adult population, older patients are often under-represented in pivotal trials. Moreover, outcomes are rarely stratified by frailty or cognitive status. Evidence specific to older adults frequently derives from subgroup or retrospective analyses, limiting the certainty of generalizability. Where robust data are available, for example hypofractionated breast RT and SBRT for inoperable lung cancer, outcomes in older cohorts are highly favorable. By contrast, evidence for modalities such as proton therapy or MR-guided RT in older populations remains limited, and conclusions should be drawn cautiously. These gaps highlight the need for future prospective trials explicitly designed to capture age-specific outcomes, encompassing not only toxicity and survival but also quality of life, independence, and preservation of functional status.
Therefore, future research priorities in geriatric radiation oncology include large-scale randomized controlled trials evaluating CGA-driven interventions and their effects on survival, functional independence, and quality of life. In addition, standardization of CGA components relevant to RT and the development of predictive models incorporating CGA data may further personalize care and enhance prognostication. Likewise, prospective randomized trials are needed to validate the predictive accuracy of frailty screening tools specifically in RT populations and to assess the impact of screening-driven interventions. The development of streamlined, technology-assisted screening processes could further facilitate routine implementation in clinical practice.
12. Conclusion
This review explored the specific considerations of RT in older adults, a rapidly growing demographic in oncology due to global aging trends. RT remains a highly effective cancer treatment in this population, offering both curative and palliative benefits across multiple cancer types. Modern RT techniques have transformed cancer care through improved accuracy, reduced toxicity, and greater adaptability to patient-specific anatomy and tumor biology. Innovations such as IMRT, SBRT, and proton therapy, supported by advanced imaging and planning systems, facilitate personalized treatment approaches. Despite these advances, evidence indicates that RT is underutilized in older adults, often due to non-medical factors such as age-related biases and systemic barriers. Addressing these disparities requires individualized treatment decisions informed by CGA, improved access to care, and increased awareness of the benefits of RT in the older population. This review emphasizes the importance of individualized treatment planning, increased adoption of frailty screening measures, and further research focused on older adults. Frailty screening tools provide valuable instruments in the RT setting, enabling early identification of older adults at risk for treatment-related complications. When combined with CGA, these tools support personalized treatment plans that balance efficacy with tolerability. As guidelines increasingly endorse frailty screening, further efforts are needed to standardize approaches and integrate them into routine oncologic care, ultimately improving outcomes for older cancer patients. Collaboration between oncologists, geriatricians, and the wider multidisciplinary team is essential to optimize treatment outcomes.
Authors contribution
O’Donovan A: Contribution of radiation therapy expertise, conception, design, interpretation of literature, drafting of manuscript and manuscript revision.
O’Hanlon S: Contribution of geriatric medicine expertise, conception, design, interpretation of literature, manuscript revision.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable. The case study included in this review is fictional and does not involve real patient data.
Consent to participate
Not applicable. No real patient data are included.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
None.
Copyright
© The Author(s) 2025.
References
-
1. Given B, Given CW. Older adults and cancer treatment. Cancer. 2008;113(S12):3505-3511.[DOI]
-
2. Shah R, Battisti NML, Brain E, Gnangnon FHR, Kanesvaran R, Mohile S, et al. Updated cancer burden in oldest old: a population-based study using 2022 Globocan estimates. Cancer Epidemiol. 2025;95:102716.[DOI]
-
3. Pilleron S, Sarfati D, Janssen-Heijnen M, Vignat J, Ferlay J, Bray F, et al. Global cancer incidence in older adults, 2012 and 2035: a population-based study. Int J Cancer. 2019;144(1):49-58.[DOI]
-
4. Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104(6):1129-1137.[DOI]
-
5. Buwenge M, Macchia G, Cavallini L, Cortesi A, Malizia C, Bianchi L, et al. Unraveling the safety of adjuvant radiotherapy in prostate cancer: impact of older age and hypofractionated regimens on acute and late toxicity - a multicenter comprehensive analysis. Front Oncol. 2023;13:1281432.[DOI]
-
6. O’Donovan A, Leech M, Gillham C. Assessment and management of radiotherapy induced toxicity in older patients. J Geriatr Oncol. 2017;8(6):421-427.
-
7. Paulsson AK, Fowble B, Lazar AA, Park C, Sherertz T. Radiotherapy utilization for patients over age 60 with early stage breast cancer. Clin Breast Cancer. 2020;20(2):168-173.[DOI]
-
8. Mackenzie P, Vajdic C, Delaney G, Comans T, Morris L, Agar M, et al. Radiotherapy utilisation rates for patients with cancer as a function of age: a systematic review. J Geriatr Oncol. 2023;14(3):101387.[DOI]
-
9. Mackenzie P, Vajdic C, Delaney G, Gabriel G, Agar M, Comans T, et al. Factors affecting radiotherapy utilisation in geriatric oncology patients in NSW, Australia. Tech Innov Patient Support Radiat Oncol. 2020;16:17-23.[DOI]
-
10. Ludmir EB, Mainwaring W, Lin TA, Miller AB, Jethanandani A, Espinoza AF, et al.. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol. 2019;5(12):1769-1773.[DOI]
-
11. O’Donovan A, O’Hanlon S. Breaking bad barriers: engaging older adults in cancer research. Age Ageing. 2024;53(2):afae019.[DOI]
-
12. Gupta T, Sinha S, Ghosh-Laskar S, Budrukkar A, Mummudi N, Swain M, et al. Intensity-modulated radiation therapy versus three-dimensional conformal radiotherapy in head and neck squamous cell carcinoma: long-term and mature outcomes of a prospective randomized trial. Radiat Oncol. 2020;15(1):218.[DOI]
-
13. Smith GL, Smith BD. Radiation treatment in older patients: a framework for clinical decision making. J Clin Oncol., 2014;32(24):2669-2678.[DOI]
-
14. Overgaard J, Alsner J, Eriksen J, Horsman MR, Grau C. Importance of overall treatment time for the response to radiotherapy in patients with squamous cell carcinoma of the head and neck. Rays. 2000;25(3):313-319.[PubMed]
-
15. Fischer AM, Hoskin PJ. Radiotherapy-induced toxicity in prostate cancer patients with hip prostheses. Radiat Oncol. 2022;17:9.[DOI]
-
16. Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630-637.[DOI]
-
17. Preziosi F, Boschetti A, Catucci F, Votta C, Vellini L, Menna S, et al. AI-driven online adaptive radiotherapy in prostate cancer treatment: considerations on activity time and dosimetric benefits. Radiat Oncol. 2025;20:116.[DOI]
-
18. Wiant D, Squire S, Liu H, Maurer J, Hayes TL, Sintay B. A prospective evaluation of open face masks for head and neck radiation therapy. Pract Radiat Oncol. 2016;6(6):e259-e267.[DOI]
-
19. Lastrucci A, Morelli I, Votta C, Maran I, Iosca N, Monaco IP. Open-face masks in radiotherapy: enhancing therapeutic strategies for head and neck and brain cancer patients—a comprehensive scoping review. Cancers. 2024;16(16):2899.[DOI]
-
20. Brunt AM, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield DJ, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395(10237):1613-1626.[DOI]
-
21. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060.[DOI]
-
22. Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385-395.[DOI]
-
23. Swaminath A, Parpia S, Wierzbicki M, Kundapur V, Faria S, Okawara GS, et al. Stereotactic vs hypofractionated radiotherapy for inoperable stage I non-small cell lung cancer: the LUSTRE phase 3 randomized clinical trial. JAMA Oncol. 2024;10(11):1571-1575.[DOI]
-
24. Prezzano KM, Ma SJ, Hermann GM, Rivers CI, Gomez-Suescun JA, Singh AK. Stereotactic body radiation therapy for non-small cell lung cancer: a review. World J Clin Oncol. 2019;10(1):14-27.[DOI]
-
25. Cummings MA, Usuki KY, Fleming FJ, Tejani MA, Katz AW. Short course radiation therapy for rectal cancer in the elderly: can radical surgery be avoided? J Gastrointest Oncol. 2019;10(2):357-361.[DOI]
-
26. Cambray M, Gonzalez-Viguera J, Berenguer MA, Macià M, Losa F, Soler G, et al. Short-course radiotherapy in locally advanced rectal cancer. Clin Transl Gastroenterol. 2020;11(6):e00162.[DOI]
-
27. François E, De Bari B, Ronchin P, Nouhaud E, Martel-Lafay I, Artru P, et al. Comparison of short course radiotherapy with chemoradiotherapy for locally advanced rectal cancers in the elderly: A multicentre, randomised, non-blinded, phase 3 trial. Eur J Cancer. 2023;180:62-70.[DOI]
-
28. McWilliams L, Farrell C, Grande G, Keady J, Swarbrick C, Yorke J. A systematic review of the prevalence of comorbid cancer and dementia and its implications for cancer-related care. Aging Ment Health. 2018;22(10):1254-1271.[DOI]
-
29. Caba Y, Dharmarajan K, Gillezeau C, Ornstein KA, Mazumdar M, Alpert N, et al. The impact of dementia on cancer treatment decision-making, cancer treatment, and mortality: a mixed studies review. JNCI Cancer Spectr. 2021;5(3):pkab002.[DOI]
-
30. Collinson M, Mason E, Kelley R, Griffiths A, Ashley L, Henry A, et al. Characteristics and general practice resource use of people with comorbid cancer and dementia in England: a retrospective cross-sectional study. BMC Prim. 2022;23:281.[DOI]
-
31. Ashley L, Surr C, Kelley R, Price M, Griffiths AW, Fowler NR, et al. Cancer care for people with dementia: Literature overview and recommendations for practice and research. CA Cancer J Clin. 2023;73(3):320-338.[DOI]
-
32. Flood J, O’Hanlon S, Gibb M, O’Donovan A. Caring for patients with dementia undergoing radiation therapy—a national audit. J Geriatr Oncol. 2019;10(5):811-818.[DOI]
-
33. McWilliams L. An overview of treating people with comorbid dementia: implications for cancer care. Clin Oncol. 2020;32(9):562-568.[DOI]
-
34. Higgins R, Spacey A, Innes A. Delivering person-centred dementia care: Perceptions of radiography practitioners within diagnostic imaging and radiotherapy departments. Dementia. 2023 Oct;22(7):1586-1603.[DOI]
-
35. Soto-Perez-de-Celis E, de Glas NA, Hsu T, Kanesvaran R, Steer C, Navarrete-Reyes AP, et al. Global geriatric oncology: achievements and challenges. J Geriatr Oncol. 2017;8(5):374-386.[DOI]
-
36. Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603.[DOI]
-
37. Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347.[DOI]
-
38. Li D, Sun CL, Kim H, Soto-Perez-de-Celis E, Chung V, Koczywas M, et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):e214158.[DOI]
-
39. Mohile SG, Mohamed MR, Xu H, Culakova E, Loh KP, Magnuson A, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398(10314):1894-1904.[DOI]
-
40. Mohile SG, Epstein RM, Hurria A, Heckler CE, Canin B, Culakova E, et al. Communication with older patients with cancer using geriatric assessment: a cluster-randomized clinical trial from the National Cancer Institute Community Oncology Research Program. JAMA Oncol. 2020;6(2):196-204.[DOI]
-
41. Lund CM, Vistisen KK, Olsen AP, Bardal P, Schultz M, Dolin TG, et al. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: a randomised trial (GERICO). Br J Cancer. 2021;124(12):1949-1958.[DOI]
-
42. Soo WK, King M, Pope A, Parente P, Darzins P, Davis ID. Integrated geriatric assessment and treatment (INTEGERATE) in older people with cancer planned for systemic anticancer therapy. J Clin Oncol. 2020;38(15_suppl):12011.[DOI]
-
43. Rostoft S, O’Donovan A, Soubeyran P, Alibhai SM, Hamaker ME. Geriatric assessment and management in cancer. J Clin Oncol. 2021;39(19):2058-2067.[DOI]
-
44. Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300.[DOI]
-
45. O’Donovan A, Leech M. Personalised treatment for older adults with cancer: the role of frailty assessment. Tech Innov Patient Support Radiat Oncol. 2020;16:30-38.[DOI]
-
46. Seghers PAL, Alibhai SM, Battisti NM, Kanesvaran R, Extermann M, O’Donovan A, et al. Geriatric assessment for older people with cancer: policy recommendations. Glob Health Res Policy. 2023;8(1):37.[DOI]
-
47. Morris L, Turner S, Thiruthaneeswaran N, Agar M. Improving the education of radiation oncology professionals in geriatric oncology: where are we and where should we be? Semin Radiat Oncol. 2022;32(2):109-114.[DOI]
-
48. VanderWalde NA, Williams GR. Developing an electronic geriatric assessment to improve care of older adults with cancer receiving radiotherapy Tech Innov Patient Support Radiat Oncol. 2020;16:24-29.[DOI]
-
49. Naseer A, Cree A, Simcock R, Jeppesen SS, Morris L, Kenis C, et al. International efforts in geriatric radiation oncology. J Geriatr Oncol. 2022;13(3):356-362.[DOI]
-
50. Skelly A, O’Donovan A. Recognizing frailty in radiation oncology clinical practice: current evidence and future directions. Semin Radiat Oncol. 2022;32(2):115-124.[DOI]
-
51. Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166-2172.[DOI]
-
52. Hamaker ME, Vos AG, Smorenburg CH, Rooij SE, Munster BC. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist. 2012;17(11):1439-1449.[DOI]
-
53. van Walree IC, Vondeling AM, Vink GR, van Huis-Tanja LH, Emmelot-Vonk MH, Bellera C, et al. Development of a self-reported version of the G8 screening tool. J Geriatr Oncol. 2019;10(6):926-930.[DOI]
-
54. DuMontier C, Loh KP, Soto-Perez-de-Celis E, Dale W. Decision making in older adults with cancer. J Clin Oncol. 2021;39(19):2164-2174.[DOI]
-
55. Leech M, Katz MS, Kazmierska J, McCrossin J, Turner S. Empowering patients in decision‐making in radiation oncology—can we do better? Mol Oncol. 2020;14(7):1442-1460.[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite