Abstract
Growth Differentiation Factor 15 (GDF15), a member of the transforming growth factor-beta superfamily, is highly expressed in response to cellular stress, ageing, and various pathological conditions. As a key component of the senescence-associated secretory phenotype, it plays important roles in modulating inflammation, mitochondrial dysfunction, energy metabolism, and appetite. It signals through the glial-derived neurotrophic factor receptor alpha-like receptor in the brainstem to suppress appetite and modulate energy balance. Increasing evidence supports that GDF15 exhibits dual and context-dependent functions in cancer, contributing to both tumor suppression and progression through its regulation of cellular proliferation, metastasis, and interactions within the tumor microenvironment. Elevated GDF15 levels have been observed in cardiovascular diseases, metabolic disorders, neurodegenerative diseases, and numerous malignancies, making it a potential biomarker and therapeutic target for a spectrum of age-related and pathological conditions, including cancer. Emerging therapeutic strategies targeting GDF15 encompass the use of agonists for obesity and antagonists for cachexia, either alone or in combination with immunotherapy, reflecting its complex role in disease. A comprehensive understanding of its context-dependent roles may shed light on fundamental disease mechanisms, offering a foundation for the development of innovative and personalized therapeutic approaches.
Keywords
1. Introduction
Growth differentiation factor 15 (GDF15), a member of the transforming growth factor-beta (TGF-β) superfamily, was first identified from a complementary DNA (cDNA) library enriched for macrophage-related genes and was subsequently named macrophage inhibitory cytokine-1 (MIC-1)[1]. In healthy human tissues, GDF15 expression is generally low, except in the placenta and prostate, which has led to alternative names such as placental transforming growth factor-beta and prostate-derived factor[2]. The glial-derived neurotrophic factor receptor alpha-like (GFRAL), part of the GFR-α receptor family within the TGF-β superfamily, currently represents the only well-characterized specific receptor for GDF15[3]. Under physiological conditions, circulating GDF15 crosses the blood-brain barrier and binds to GFRAL in the area postrema and nucleus of the solitary tract in the brainstem, where it suppresses appetite[4]. Metformin, the first-line therapy for type 2 diabetes, exerts its weight-lowering effect through kidney-derived GDF15[5]. Moreover, GDF15 is a key molecule for embryo implantation and survival, yet it is simultaneously a major contributor to pregnancy-associated nausea and vomiting[5].
Notably, possibly distinct from the classical GDF15-GFRAL signaling pathway, GDF15 expression is frequently upregulated in various conditions, including diverse tumors[6-8], infections and sepsis[9], as well as numerous age-related diseases such as cardiovascular disorders[10] and chronic kidney and lung diseases[11]. As a key factor secreted by senescent cells exhibiting the senescence-associated secretory phenotype (SASP)[12], GDF15 emerges as the focus of this review, particularly regarding its role in chronic low-grade inflammation and the resulting immunosuppression and tumor-associated immunosenescence.
2. Chronic Inflammation-Driven GDF15 Induction Links Ageing to Age-Related Diseases
2.1 GDF15 as a hallmark of ageing and senescence
Recent evidence have highlighted a strong association between GDF15 and ageing. A cohort study showed that serum GDF15 levels, similar to telomere length, can independently predict all-cause mortality regardless of genetic background[13]. In line with this, elevated circulating GDF15 in older adults has been linked to monocyte dysfunction, suggesting a role in immune decline during ageing[14]. Mechanistically, GDF15 is upregulated in chronic inflammation and implicated in various age-related diseases. A comprehensive multi-tissue transcriptomic analysis further identified it as a key aging biomarker with high predictive value across tissues, particularly in cardiometabolic organs such as the heart, liver, skeletal muscle, and adipose tissue, where its levels correlate with clinical indicators of metabolic health[15].
2.2 Transcriptional regulation of GDF15 in senescence
Multiple cellular stress signals—including amino acid deprivation, hypoxia, mitochondrial stress, and endoplasmic reticulum stress—activate activating transcription factor 4 (ATF4). ATF4 forms a heterodimer with CCAAT/enhancer-binding protein homologous protein (CHOP) via phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α), enhancing GDF15 transcription[16,17]. Notably, hypoxia can induce GDF15 expression through CHOP-dependent mechanisms independent of p53 or hypoxia-inducible factor 1 (HIF-1)[18].
The GDF15 promoter region contains binding sites for several transcription factors, including specificity protein 1, early growth response protein 1 (Egr-1), p53, and chicken ovalbumin upstream promoter transcription factor 1 (COUP-TF1)[19,20]. Furthermore, peroxisome proliferator-activated receptor γ (PPARγ) ligands enhance GDF15 expression via interactions with Egr-1 and ATF-3/4[21,22]. In cardiovascular disease, C-reactive protein stimulates GDF15 expression in endothelial cells through the p53 pathway[23].
However, the potential mechanism of GDF15 functions in cellular senescence still needs further exploration. Park et al. reported that GDF15 expression significantly increased in human aortic endothelial cells (HAECs) following exposure to ionizing radiation; viral overexpression of GDF15 in HAECs induced the generation of reactive oxygen species (ROS), triggering senescence through the ERK/p16 signaling pathway[24].
2.3 Organelle-specific context of GDF15 function
2.3.1 GDF15 as a key mediator of mitochondrial stress response and homeostasis
Mitochondrial dysfunction is a hallmark of cellular senescence and plays a critical role in ageing process[25,26]. Under stress, misfolded proteins accumulate within mitochondria and activate the mitochondrial unfolded protein response (UPR), which upregulates nuclear-encoded chaperones and mitokines such as GDF15[27]. In cases of impaired oxidative phosphorylation, CHOP-dependent transcription further enhances GDF15 expression[28]. Functionally, GDF15 helps maintain mitochondrial homeostasis by stabilizing membrane potential, reducing oxygen consumption, limiting mitochondrial DNA release, and alleviating damage[29-31] (Table 1).
| Regulatory Mechanism | Key Molecule Pathways | Biological Effects | Pathological Significance |
| Energy Metabolism | Activation of AMPK-PGC-1α pathway | ↑ Fatty acid oxidation, ↓ Gluconeogenesis | Ameliorates NAFLD/NASH |
| Antioxidant Defense | Upregulation of SLC7A11/GPX4 axis | ↑ Glutathione synthesis, ↓ Lipid peroxidation | Inhibits ferroptosis; Combats chemoresistance |
| Mitochondrial Dynamics | Regulation of MFN2/DRP1 balance | Balanced mitochondrial fusion/fission | Maintains mitochondrial network integrity |
| Mitochondrial Quality Control | Enhancement of PINK1/Parkin pathway | ↑ Mitophagy, Clearance of damaged mitochondria | Neuroprotection; Delays ageing |
GDF15: growth differentiation factor 15; AMPK-PGC-1α: AMP-activated protein kinase-peroxisome proliferator-activated receptor gamma coactivator 1-alpha; ↑: increased; ↓: decreased; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; SLC7A11: solute carrier family 7 member 11; GPX4: glutathione peroxidase 4; MFN2: mitofusin 2; DRP1:dynamin-related protein 1; PINK1: PTEN-induced kinase 1.
A variety of mitochondrial stressors, including electron transport chain defects, mtDNA mutations, and proteostasis imbalance, can robustly induce GDF15[32,33]. In mitochondrial myopathy models, GDF15 expression increases over tenfold in response to mtDNA deletions, correlating with the level of stress response activation[33,34]. Mechanistically, mitochondrial dysfunction triggers the integrated stress response and UPR, particularly via the eIF2α-ATF4/ATF5-CHOP pathway, which directly promotes GDF15 transcription[34,35].
The functional significance of GDF15 is evident in knockout models. GDF15-deficient fibroblasts show impaired oxidative phosphorylation, increased reactive oxygen species, and decreased membrane potential[36]. In endothelial cells, its absence accelerates ageing phenotypes such as increased senescence markers and reduced proliferation[24]. In mice with muscle-specific mitochondrial dysfunction, elevated tissue and circulating GDF15 levels indicate its role as a systemic stress signal[33,37].
Beyond its role in stress signaling, GDF15 supports mitochondrial metabolic regulation. In hepatocytes, it activates AMP-activated protein kinase-Peroxisome proliferator-activated receptor gamma coactivator 1-alpha signaling to enhance fatty acid oxidation and reduce lipid accumulation[38]. In adipose tissue, it promotes browning and increases energy expenditure via mitochondrial uncoupling[39].
GDF15 also regulates mitochondrial morphology and quality control. It modulates the Mitofusin 2/Dynamin-Related Protein 1 balance to maintain network integrity and reverses pathological mitochondrial fission in NASH models[40,41]. Moreover, it promotes mitophagy through the PTEN-induced kinase 1/Parkin pathway, enhancing the clearance of damaged mitochondria and limiting apoptotic signaling, especially in ageing tissues[42,43].
Collectively, these findings highlight GDF15 as both a sensor and effector of mitochondrial stress, linking organellar dysfunction to systemic ageing responses. Its multifaceted roles in mitochondrial maintenance, energy metabolism, and quality control underscore its potential as a therapeutic target in age-related metabolic decline.
2.3.2 GDF15 interaction with endoplasmic reticulum (ER) stress
The ER, a critical organelle for protein synthesis, folding, and modification, undergoes the UPR upon functional disturbance, which is associated with various diseases. Research finds that GDF15 plays an important role in alleviating ER stress. In adipose tissue, neurotensin-neurotensin receptor 2 (NTS-NTSR2) signaling regulates ceramide metabolism via GDF15, impacting ER stress levels. Specifically, NTSR2 deletion leads to the accumulation of ceramides C20~C24, activating the PERK-eIF2α-ATF4 pathway and inducing ER stress; conversely, GDF15 can reduce ceramide levels and alleviate the stress state[44].
In the context of liver pathology, ER stress and mitochondrial dysfunction create a vicious cycle. Hepatocytes in non-alcoholic fatty liver disease (NAFLD) patients exhibit both ER stress and mitochondrial dysfunction. Glucagon-like peptide-1 (GLP-1) receptor agonists (such as liraglutide) significantly improve ER stress in hepatocytes by inducing GDF15 expression[45,46]. Clinical studies show that NAFLD patients with diabetes treated with liraglutide for 26 weeks exhibit significant increases in serum GDF15 levels, reductions in intrahepatic lipid (IHL) content, and an inverse correlation between changes in GDF15 and IHL[47]. Relevant literature reports that GDF15 deficiency diminishes the protective effect of the GLP-1 analog Exendin-4 against palmitate-induced ER stress[48].
These findings position GDF15 as a crucial mediator between ER stress and metabolic regulation. By modulating lipid signaling and stress response pathways, GDF15 contributes to cellular resilience in metabolic disorders such as NAFLD.
2.3.3 GDF15 regulation of lysosomal function
Lysosomes, as cellular degradation centers, exhibit a close interplay between their biogenesis/function and GDF15. Studies indicate a bidirectional regulatory relationship between the transcription factor, transcriptional factor EB (TFEB) (the master regulator of lysosomal biogenesis), and GDF15[49]. Under lysosomal stress conditions, TFEB is activated and translocated to the nucleus, where it directly binds to the GDF15 promoter region, inducing its expression[49,50]. Conversely, GDF15 influences TFEB activity and lysosome biogenesis via the mTORC1 signaling pathway. In obesity models, GDF15 treatment increases TFEB nuclear translocation in the liver, elevates lysosome numbers, and enhances autophagic-lysosomal pathway function[49,51].
2.3.4 GDF15 regulation of nuclear gene expression
In addition to its role as a secreted protein, GDF15 also exerts nuclear functions via its precursor form harbouring a nuclear localization signal that facilitates its translocation into the nucleus, directly influencing gene expression[52]. Research has found that the GDF15 precursor protein accumulates within the nuclei of various cell types, interacting with histone-modifying enzymes to regulate stress-response-related genes. In senescent cells, levels of the nuclear GDF15 precursor protein increase before forming a complex with p53, enhancing the expression of senescence-associated genes like p21[53].
2.4 Diverse functions of GDF15 in senescence and age-related diseases
A number of studies has demonstrated that increased GDF15 is critically involved in various age-related diseases, including heart failure[54], Alzheimer disease[55], sarcopenia[56], diabetes[57] and even psychosocial disorders[58,59]. At present, numerous studies are using large-scale proteomics (plasma) to identify inflammation-related markers in these diseases, with particular attention to GDF15 as a potential predictor of diseases progression and clinical outcomes. Several models have been established in this context[60-65]. Table 2 summarizes the clinical value of GDF15 in common age-related diseases.
| Age-related diseases | GDF15 cutpoints | Clinical Significance | Reference |
| Atherosclerotic cardiovascular disease | < 1,200 ng/L 1,200-1,800 ng/L > 1,800 ng/L | increased rate of cardiovascular death | [58] |
| Chronic heart failure | < 2,000 ng/L > 2,000 ng/L | increased rate of all-cause mortality | [66] |
| Alzheimer disease dementia | / | increased rate of all-cause dementia | [67] |
| Dementia | / | increased rate of all-cause dementia | [68] |
| Sarcopenia and frailty | < 1,541 ng/L > 2,166 ng/L | Diagnostics | [67] |
| Frailty | / | Diagnostics | [68] |
GDF15: growth differentiation factor 15.
Recent studies also suggested that GDF15 may function as an upstream regulatory molecule of sirtuin 1 (SIRT1), a trigger of ageing related pathways[69]. Cell senescence concludes the poor fracture prognosis and may even lead to muscle atrophy. Falvino et al. showed that SASP (GDF15, IL6, TNF-α) is increased while SIRT1 is down-expressed in fractured patients, indicating a potential correlation between GDF15 and SIRT1 in cell senescence[70]. Moreover, Cheng et al. identified GDF15 as a mediator of aged perivascular adipose tissue (PVAT), which can increase risks of cardiovascular diseases[71]. Aged PVAT, compared to young PVAT, can enhance the paracrine of GDF15, which down-regulates the AMPK/SIRT1 signaling in aortas, thus activating the vascular ageing hallmarks.
These results brought by GDF15 appear to be more pronounced in young vasculature that are treated. In addition, Xiong, W.P. et al. demonstrated that destabilization of GDF15 mRNA by circLPAR1 activates the SIRT1/Nrf-2/HO-1 axis, thus exacerbating inflammation and oxidative stress in Alzheimer’s diseases[72]. The GDF15 regulatory effect of Nrf-2 is validated in spinal cord injury[73]. GDF15 has also been shown to inhibit neuronal ferroptosis through p62-dependent Keap1-Nrf2 pathway, and silencing and knocking down GDF15 will enhance the neuroinflammation. Interestingly, evidence suggests a bidirectional regulatory relationship between GDF15 and Nrf2. Satta et al. reported that the Nrf2-OSGIN1&2-HSP70 pathway in coronary artery endothelial cells will be activated after prolonged exposure to cigarette smoke extract and tumor necrosis factor alpha (TNF-α), hence upregulating the GDF15 expression[74]. The protective role of GDF15 has also been validated in acute lung injury and hypoxia-reoxygenation-induced vascular damages, which are not aged-related diseases[75,76].
An intriguing aspect of GDF15 biology lies in its multiple diverse effects under different circumstances[77], which may be attributed to the differential activation of SIRT1 related pathways. The receptor of GDF15 may be the key which SIRT1 related pathway will be activated[77]. The multifaceted roles of GDF15 are exhibited in the various complications of diabetes. In the research of Almohaimeed et al., treating the rats with diabetic cardiomyopathy with metformin can increase the expression of Klotho and GDF15 by shifting the macrophages polarization[78]. Researchers believe that despite elevated GDF15 levels in diabetic patients compared to non-diabetic individuals, GDF15 may be involved in cell survival and tissue repair by exhibiting a cardioprotective effect. In another study, Alonazi et al. used liposomal clodronate to treat rats with diabetic nephropathy and observed a downregulation of inflammatory and oxidative makers, including GDF15, followed by the macrophage polarization towards the anti-inflammatory phenotype[79].
The expression pattern of GDF15 may keep varying[80]. Regional variations in GDF15 expression have been observed across different brain areas, and differences in GDF15 protein maturation have been reported in patients with Alzheimer’s disease. Although anti-GDF15 therapy has showed the surprising outcomes in recent clinical trials for cancer cachexia[81,82], the unclear role of GDF15 in cellular senescence calls for a comprehensive reevaluation of GDF15-based treatment strategies. The diverse functions of GDF15 raise the question of what determines its specific role in senescence. In other words, GDF15 expression is not simply a matter of upregulation or downregulation; instead, it requires in-depth investigation across various age-related diseases and stages of disease progression. This complexity poses significant challenges for the use of GDF15 as a reliable biomarker for disease progression and prognosis, as well as for its development as a potential therapeutic target.
Inflammation induced by various stressors promotes the production of GDF15, which primarily acts through the GFRAL-RET co-receptor to activate classical pathways such as MAPK/ERK, PI3K/AKT, and PLCγ/PKC. Activation of these pathways can influence neuronal activity and satiety signaling, regulate metabolism, and ultimately lead to muscle atrophy, fat loss, and frailty. Concurrently, these pathways also promote proliferation and migration in tumors. However, unlike the well-defined GFRAL-RET co-receptor, the specific receptor mediating the effects of GDF15 in tumors remains unclear (Figure 1).
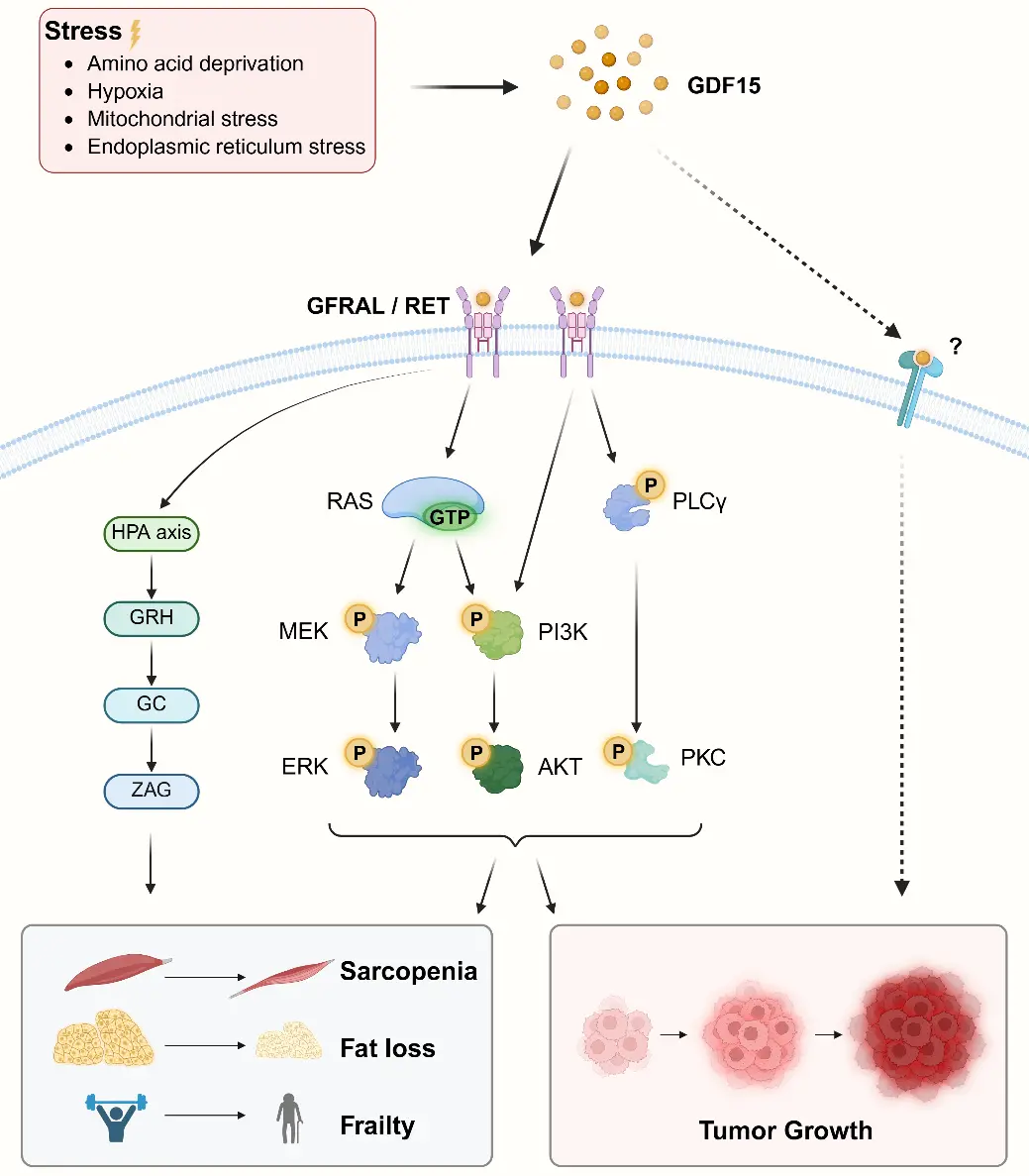
Figure 1. Classical pathways activated by GDF15. Created in https://BioRender.com. GDF15: growth differentiation factor 15; GFRAL: glial-derived neurotrophic factor receptor alpha-like; RET:Rearranged during transfection ; RAS: Rat sarcoma virus oncogene; GTP: guanosine triphosphate; PLCy: phospholipase C gamma; ERK: extracellular signal-regulated kinase; AKT: protein kinase B; PKC: protein kinase C.
3. GDF15 as a Potential Target to Overcome Tumor Therapy Resistance
3.1 GDF15 displays dual activities in cancer biology
Some of the pathways through which GDF15 exerts its effects in tumors overlap with those involved in metabolic regulation and cellular senescence (Figure 1). In specific contexts, GDF15 may exhibit anti-tumorigenic properties by inducing apoptosis, thereby suppressing tumor initiation and growth. For instance, in colorectal cancer, GDF15 acts as a tumor suppressor by inhibiting the β-catenin and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways through interaction with epithelial cell adhesion molecule (EpCAM)[83]. However, in advanced-stage cancers, plasma levels of GDF15 are markedly elevated, which facilitates tumor invasion and metastasis. GDF15 promotes the proliferation and dissemination of pancreatic ductal adenocarcinoma (PDAC) via the TGFβ-Smad2/3 signaling pathway[84]. Conversely, GDF15 depletion has been shown to inhibit cervical cancer progression by suppressing the same TGFβ-Smad2/3 axis[85]. Similarly, GDF15 inhibition has been reported to slow gastric cancer progression by modulating the STAT3 pathway, thereby blocking epithelial-mesenchymal transition (EMT)[86]. Recent studies show that GDF15 upregulates key glycolytic gene-glucose transporter 1 (GLUT1), promoting the Warburg effect; correspondingly, GDF15 knockdown markedly suppresses tumor growth, while its overexpression accelerates progression[87]. In most cases, GDF15 is considered to promote cancer progression, particularly in advanced-stage tumors. The observed anti-tumor effects of GDF15 could be related to the molecular subtype evolution during the transition from early- to late-stage disease, or to the heterogeneity across different cancer types.
3.2 GDF15 is involved in tumor angiogenesis.
The pro-angiogenic role of GDF15 is primarily observed in ischemic heart disease, where it contributes to reduced mortality and improved clinical outcomes[88]. However, angiogenesis supports tumor growth and provides a potential route for metastasis, making anti-angiogenic therapy widely applied in the treatment of various tumors[89]. In human umbilical vein endothelial cells (HUVECs), GDF15 promotes angiogenesis via the HIF-1α/vascular endothelial growth factor (VEGF)-dependent signaling pathway. Knockdown of hypoxia-inducible factor-1α (HIF-1α) using small interfering RNA (siRNA) abolishes GDF15-mediated angiogenic effects and suppresses VEGF expression[90]. In glioblastoma multiforme (GBM), radiation-induced GDF15 is essential for the cross-talk between endothelial cells and GBM cells and promotes angiogenesis[91]. In hepatocellular carcinoma (HCC), the pro-angiogenic role of GDF15 is mediated through activation of proto-oncogene tyrosine-protein kinase Src (Src) and its downstream effectors AKT, MAPK, and NF-κB, which can be inhibited by thalidomide[92].
3.3 GDF15 contributes to chemotherapy resistance
Chemotherapy has been shown to induce GDF15 secretion from human pancreatic stellate cells, thereby contributing to chemoresistance in PDAC[93]. In breast cancer, GDF15 promotes drug resistance through activation of the FOXM1 pathway[94]. Notably, GDF15 secretion occurs specifically during the drug-tolerant persistence phase and is absent in cells sensitive to eribulin, a chemotherapeutic agent used for advanced breast cancer. Further studies have demonstrated that combining eribulin with GDF15 inhibitors enhances treatment efficacy in breast cancer[95]. GDF15 post-transcriptionally regulates Nrf2 protein stability via the canonical PI3K/AKT/GSK3β signaling pathway, while Nrf2, in turn, acts as a transcription factor to promote GDF15 expression, forming a positive feedback loop that maintains redox homeostasis and contributes to oxaliplatin resistance in colorectal cancer (CRC)[96]. As a metabolic regulatory factor, GDF15 can also promote chemoresistance through metabolic modulation. For example, in esophageal squamous cell carcinoma (ESCC), GDF15 enhances resistance to cisplatin by upregulating the expression of UDP-glucuronosyltransferase 1A (UGT1A) family enzymes[97]. Additionally, GDF15 modulates ferroptosis-related pathways, protecting cancer cells from ROS-induced damage and thus supporting their survival under therapeutic stress; inhibition of GDF15, however, enhances the sensitivity of MSI-H CRC to 5-FU therapy[98]. Molecular mechanism studies show that GDF15 can reverse the MTX-induced downregulation of SLC7A11/GPX4, decrease the expression of ferroptosis marker proteins ACSL4 and PRDX3, and elevate SLC7A11 mRNA levels[99].
3.4 GDF15 plays a key role in the tumor immune microenvironment
3.4.1 GDF15-mediated immunosuppressive pathways in ageing and immunosenescence
Ageing is associated with increased immunosuppression and immunosenescence. Several studies have demonstrated significant age-related increases in immunosuppressive cells-such as myeloid-derived suppressor cells, regulatory T cells (Tregs), and M2 macrophages-in the circulation, immune organs, and peripheral tissues of both humans and rodents[100,101]. Senescent cells evade immune surveillance partly through the upregulation of inhibitory immune checkpoint ligands on T cells, NK cells, and macrophages[102,103]. For example, programmed death-ligand 1 (PD-L1) expression increases in multiple tissues during ageing, enabling senescent pulmonary fibroblasts to escape PD-1⁺ cytotoxic T cells[103]. GDF15, secreted abundantly by senescent cells[12], contributes to this immunosuppressive environment. GDF15 induces PD-L1 expression[104], thereby facilitating immune evasion and sustaining senescent cell survival in aged tissues. Notably, GDF15 has been identified as a component of SASP. During early tumorigenesis or initial therapeutic interventions, SASP factors such as IL-1α, IL-6, CCL2, and CXCL10 may enhance local inflammatory responses and promote immune clearance. In contrast, at advanced stages or during therapeutic resistance, SASP components including TGF-β and IL-10 facilitate the establishment of an immunosuppressive TME through multiple mechanisms[104], with GDF15 implicated as a participant in this process.
Although the exact mechanisms remain unclear, one proposed pathway involves GDF15-mediated activation of the hypothalamic-pituitary-adrenal (HPA) axis. GDF15 stimulates glucocorticoid secretion via GFRAL signaling, as evidenced by studies in mice where GFRAL blockade prevented this response[105]. Additionally, as a non-specific inducer of TGF-β signaling, GDF15 may exert a significant portion of its immunosuppressive effects through TGF-β-dependent pathways.
Unlike established immune checkpoints such as PD-1/PD-L1 and CTLA-4, which are membrane-bound proteins, GDF15 is a secreted protein, with its functions potentially more complex than those of traditional immune checkpoints due to its capacity for distal effects and systemic actions of secreted proteins. Other studies have indicated that GDF15 exerts multifaceted roles both intracellularly and extracellularly, further increasing the challenge of elucidating its detailed and specific mechanisms[105].
3.4.2 GDF15 promotes Treg expansion and function
GDF15 signaling enhances the generation and functional capacity of Tregs via multiple mechanisms, including the upregulation of indoleamine 2,3-dioxygenase (IDO), which induces FoxP3 expression and promotes Treg development[106]. Furthermore, it drives the conversion of naïve CD4+ T cells into induced Tregs (iTregs) by engaging CD48 and inhibiting the ERK-AP-1 pathway downstream of the TCR[107]. Unlike TGF-β, GDF15 does not alter FOXP3 mRNA levels but prevents FOXP3 ubiquitination, thereby enhancing the suppressive function of natural Tregs (nTregs) and supporting iTreg induction[108]. Multiple studies confirm that GDF15 promotes Treg-mediated suppression of conventional T cells[43].
3.4.3 GDF15 suppresses dendritic cells maturation and antigen presentation
Dendritic cells (DCs) serve as pivotal initiators of antigen-specific immune responses. GDF15 impairs DC function by inhibiting surface protrusion formation, and downregulating maturation markers and co-stimulatory molecules thereby limiting T cell and cytotoxic T lymphocyte (CTL) activation[109]. In ovarian cancer, GDF15 targets CD44 on DCs, weakening their immunostimulatory capacity and promoting immune evasion[110]. Similarly, in HBV-related HCC, elevated GDF15 suppresses DC-mediated antigen presentation, contributing to recurrence and poor prognosis[111].
3.4.4 GDF15 modulates tumor-associated macrophage (TAM) phenotype and function
Tumor hypoxia boosts the recruitment and polarization of GDF15-secreting TAMs, enhancing chemoresistance in CRC cells[112]. In HCC, tumor-derived GDF15 aids immune in escaping by inhibiting the TAK1-NF-κB pathway in macrophages, lowering TNF and nitric oxide production, and weakening macrophage activity[113,114]. Besides, GDF15 drives macrophage polarization to the M2 phenotype, supporting tumor proliferation and invasion, and alleviating metabolic syndrome features[115,116]. In esophageal squamous cell carcinoma (ESCC), recombinant GDF15 promotes tumor growth via activating intracellular PI3K/AKT and MEK/ERK signaling pathways, and increasing macrophage infiltration directed by cancer-transformed normal cells[117].
3.4.5 GDF15 impairs natural killer (NK) cells and cytotoxic CD8+ T cells Mediated Immunity
Natural killer (NK) cells and CD8+ T cells cytotoxicity constitutes a critical component of anti-tumor immunity[118]. In colon cancer, GDF15 promotes immune evasion by reducing NK cell cytotoxicity through acetylation-dependent regulation of its expression[119]. Similarly, GDF15 is overexpressed in malignant gliomas and contributes to NK cell evasion, although the mechanisms remain to be fully elucidated[120]. Moreover, GDF15 impairs the trafficking of cytotoxic CD8+ T cells by disrupting LFA-1/β2-integrin-mediated adhesion to activated endothelial cells, which is essential for T cell infiltration into tissues. This effect has been confirmed through in vitro experiments, mouse models, and human melanoma samples[121]. These findings highlight the potential of anti-GDF15 therapies to improve antitumor immunity. The role of GDF15 in regulation of cancer and the microenvironment is briefly illustrated (Figure 2).
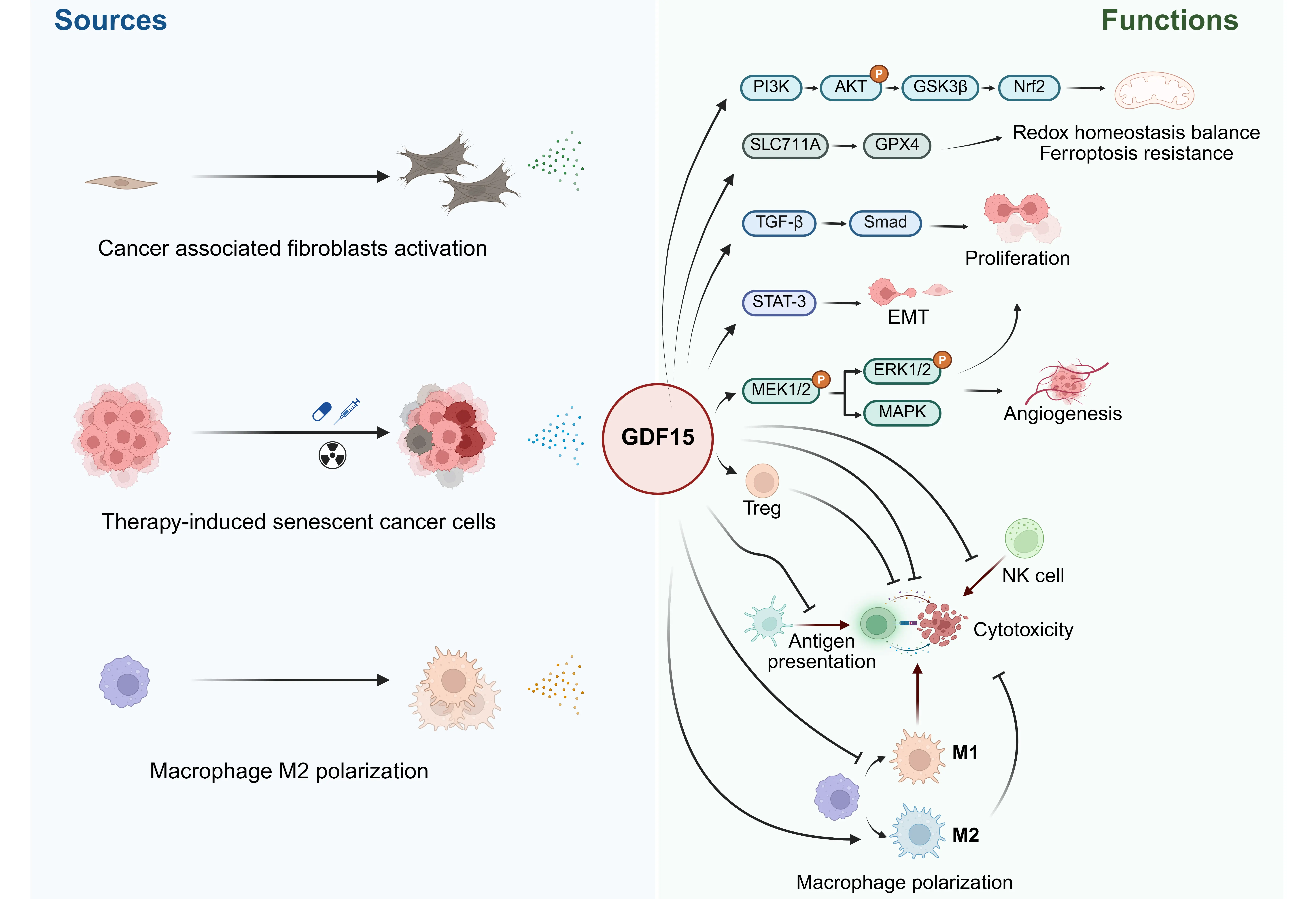
Figure 2. The sources and functions of GDF15 in tumor microenvironment. Created in https://BioRender.com. GDF15: growth differentiation factor 15; PI3K: Phosphoinositide 3-Kinase; AKT: Protein Kinase B; GSK3β: glycogen synthase kinase 3; Nrf2: Nuclear factor E2-related factor 2; GPX4: glutathione peroxidase 4; TGF-β: transforming growth factor-beta; STAT-3: signal transducer and activator of transcription 3; EMT: epithelial-mesenchymal transition; MAPK: mitogen-activated protein kinase.
GDF15 in the tumor microenvironment may originate from tumor cells, cancer-associated fibroblasts (CAFs), or immune cells such as M2 macrophages. GDF15 exerts a complex regulatory role within the tumor microenvironment, contributing to tumor growth, angiogenesis, and metastasis. It also promotes therapeutic resistance by mediating ferroptosis resistance, facilitating EMT, and negatively regulating immune responses (Figure 2).
4. Conclusion and Perspective
GDF15 exhibits pleiotropic biological functions, playing roles in ageing, pregnancy, stress response, immunosuppression, and various chronic diseases, although some findings remain contradictory. As our growing insight into its physiological and pathological roles deepens, GDF15 is increasingly regarded as a promising therapeutic target for metabolic disorders and malignancies, even though most related therapies remain in clinical trial phases.
The long-acting receptor agonist LY3463251 (NCT03764774) has shown appetite and food intake suppression with modest weight loss, independent of nausea and emesis[122]. Conversely, as previously noted, GDF15 antagonists have shown efficacy in ameliorating cancer cachexia[81,82]. Recent clinical trials increasingly explore GDF15-neutralizing antibodies as components of anti-cancer therapy in advanced malignancies, beyond their traditional role in managing cachexia. In 2025, the first-in-human phase I/IIa study (GDFATHER-1/2a trial, NCT04725474)[123] evaluated the use of the neutralizing anti-GDF15 antibody visugromab (CTL-002) in combination with the anti-PD-1 antibody nivolumab in patients with advanced cancers that were refractory to prior anti-PD-1 or anti-PD-L1 therapy. In the phase I cohort and phase IIa cohorts for non-small cell lung cancer (NSCLC) and urothelial carcinoma (UC), objective responses were observed in 4/25 (16%), 4/27 (14.8%), and 5/27 (18.5%) patients, respectively. In contrast, another phase I/IIa open-label study (NCT05397171)[124] assessing AZD8853 monotherapy showed no objective responses in solid tumor patients; only 5 out of 16 patients (31.3%) achieved stable disease, suggesting that monotherapy targeting GDF15 alone may not be the optimal strategy. GDF15 antagonists, as novel anti-tumor therapeutics, still face several challenges. First, although two clinical studies have indicated a basic safety profile, the limited sample sizes of these phase I/IIa trials may not have revealed all potential risks. Second, regarding efficacy, while numerous preclinical studies from different laboratories have reported promising anti-tumor effects of GDF15 inhibition, clinical trials suggest that these effects may be overestimated; GDF15 blockade might serve only as a sensitizing strategy to enhance the efficacy of immunotherapy or conventional chemoradiotherapy. In future clinical practice, patient selection, potentially guided by tissue or liquid biopsy, may help identify those who could benefit most from GDF15-targeted therapy, such as patients with high GDF15 expression levels.
Collectively, current evidence underscores the multifaceted roles of GDF15 and highlights its therapeutic potential across diverse pathological contexts. Nonetheless, rigorous mechanistic investigations and biomarker-guided clinical trials will be essential to delineate its optimal applications, thereby advancing GDF15 modulation from an emerging concept toward a clinically actionable strategy.
Authors contribution
Lyu Z, Deng P, Huang Q: Writing-original draft.
Fan N, Bruns C: Review & editing.
Zhao Y: Conceptualization, review & editing.
Conflicts of interest
The authors declared that there are no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
References
-
1. Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci. 1997;94(21):11514-11519.[DOI]
-
2. Lawton LN, de Fatima Bonaldo M, Jelenc PC, Qiu L, Baumes SA, Marcelino RA, et al. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene. 1997;203(1)17-26:[DOI]
-
3. Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017;23(10):1150-1157.[DOI]
-
4. Zhang SY, Bruce K, Danaei Z, Li RJ, Barros DR, Kuah R, et al. Metformin triggers a kidney GDF15-dependent area postrema axis to regulate food intake and body weight. Cell Metab. 2023;35(5):875-886.[DOI]
-
5. Yang G, Yao G, Wang H, Jiang R, Fang J, Hu J, et al. Melatonin affects trophoblast epithelial-to-mesenchymal transition and oxidative damage resistance by modulating GDF15 expression to promote embryo implantation. Commun Biol. 2025;8(1):396.[DOI]
-
6. Myojin Y, Hikita H, Sugiyama M, Sasaki Y, Fukumoto K, Sakane S, et al. Hepatic stellate cells in hepatocellular carcinoma promote tumor growth via growth differentiation factor 15 production. Gastroenterology. 2021;160(5):1741-1754.[DOI]
-
7. Wang Z, Wang S, Jia Z, Hu Y, Cao D, Yang M, et al. YKL-40 derived from infiltrating macrophages cooperates with GDF15 to establish an immune suppressive microenvironment in gallbladder cancer. Cancer Lett. 2023;563:216184.[DOI]
-
8. Zhao J, Li Y, Huang Y, Su P, Nie F, Yang P, et al. Tumor-derived GDF15 induces tumor associated fibroblast transformation from BMSCs and fibroblasts in oral squamous cell carcinoma. J Cell Physiol. 2025;240(1):e31498.[DOI]
-
9. Li X, Sun H, Zhang L, Liang H, Zhang B, Yang J, et al. GDF15 attenuates sepsis-induced myocardial dysfunction by inhibiting cardiomyocytes ferroptosis via the SOCS1/GPX4 signaling pathway. Eur J Pharmacol. 2024;982:176894.[DOI]
-
10. Kato ET, Morrow DA, Guo J, Berg DD, Blazing MA, Bohula EA, et al. Growth differentiation factor 15 and cardiovascular risk: individual patient meta-analysis. Eur Heart J. 2023;44(4):293-300.[DOI]
-
11. Lasaad S, Crambert G. GDF15, an emerging player in renal physiology and pathophysiology. Int J Mol Sci. 2024;25(11):5956.[DOI]
-
12. Evans DS, Young D, Tanaka T, Basisty N, Bandinelli S, Ferrucci L, et al. Proteomic analysis of the senescence-associated secretory phenotype: GDF-15, IGFBP-2, and Cystatin-C are associated with multiple aging traits. J Geronto A Biol Sci Med Sci. 2024;79(3):glad265.[DOI]
-
13. Wiklund FE, Bennet AM, Magnusson PK, Eriksson UK, Lindmark F, Wu L, et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15): A new marker of all-cause mortality. Aging Cell. 2010;9(6):1057-1064.[DOI]
-
14. Pence BD, Yarbro JR, Emmons RS. Growth differentiation factor-15 is associated with age-related monocyte dysfunction. Aging Med. 2021;4(1):47-52.[DOI]
-
15. Arif M, Lehoczki A, Haskó G, Lohoff FW, Ungvari Z, Pacher P. Global and tissue-specific transcriptomic dysregulation in human aging: Pathways and predictive biomarkers. GeroScience. 2025;47:5917-5936. [Doi: 10.1007/s11357-025-01672-z]
-
16. Al-Mudares F, Reddick S, Ren J, Venkatesh A, Zhao C, Lingappan K. Role of growth Differentiation Factor 15 in lung disease and senescenceet al. Role of growth Differentiation Factor 15 in lung disease and senescence: Potential role across the lifespan. Front Med. 2020;7:594137. [Doi: 10.3389/fmed.2020.594137]
-
17. Wang D, Day EA, Townsend LK, Djordjevic D, Jørgensen SB, Steinberg GR. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. 2021;17(10):592-607. [Doi: 10.1038/s41574-021-00529-7]
-
18. Zheng H, Wu Y, Guo T, Liu F, Xu Y, Cai S. Hypoxia induces growth differentiation factor 15 to promote the metastasis of colorectal cancer via PERK‐eIF2α signaling. Biomed Res Int. 2020;2020(1):5958272.[DOI]
-
19. Böttner M, Laaff M, Schechinger B, Rappold G, Unsicker K, Suter-Crazzolara C. Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1). Gene. 1999;237(1):105-111.[DOI]
-
20. Baek SJ, Horowitz JM, Eling TE. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Biol Chem. 2001;276(36):33384-33392.[DOI]
-
21. Yang H, Choi HJ, Park SH, Kim JS, Moon Y. Macrophage inhibitory cytokine-1 (MIC-1) and subsequent urokinase-type plasminogen activator mediate cell death responses by ribotoxic anisomycin in HCT-116 colon cancer cells. Biochem Pharmacol. 2009;78(9):1205-1213.[DOI]
-
22. Li A, Zhao F, Zhao Y, Liu H, Wang Z. ATF4-mediated GDF15 suppresses LPS-induced inflammation and MUC5AC in human nasal epithelial cells through the PI3K/Akt pathway. Life Sci. 2021;275:119356.[DOI]
-
23. Kim Y, Noren Hooten N, Evans MK. CRP stimulates GDF15 expression in endothelial cells through p53. Mediators Inflamm. 2018;2018(1):8278039.[DOI]
-
24. Park H, Kim CH, Jeong JH, Park M, Kim KS. GDF15 contributes to radiation-induced senescence through the ROS-mediated p16 pathway in human endothelial cells. Oncotarget. 2016;7(9):9634-9644.[DOI]
-
25. Yatsuga S, Fujita Y, Ishii A, Fukumoto Y, Arahata H, Kakuma T, et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann Neurol. 2015;78(5):814-823 (2015).[DOI]
-
26. Morrow RM, Picard M, Derbeneva O, Leipzig J, McManus MJ, Gouspillou G, et al. Mitochondrial energy deficiency leads to hyperproliferation of skeletal muscle mitochondria and enhanced insulin sensitivity. Proc Natl Acad Sci. 2017;114(10):2705-2710.[DOI]
-
27. Rochette L, Meloux A, Zeller M, Malka G, Cottin Y, Vergely C. Mitochondrial SLC25 carriers: Novel targets for cancer therapy. Molecules. 2020;25(10):2417.[DOI]
-
28. Kang SG, Choi MJ, Jung SB, Chung HK, Chang JY, Kim JT, et al. Differential roles of GDF15 and FGF21 in systemic metabolic adaptation to the mitochondrial integrated stress response. iScience. 2021;24(3):102181.[DOI]
-
29. Liu H, Liu J, Si L, Guo C, Liu W, Liu Y. GDF-15 promotes mitochondrial function and proliferation in neuronal HT22 cells. J Cell Biochem. 2019;120(6):10530-10547.[DOI]
-
30. Wang Y, Chen C, Chen J, Sang T, Peng H, Lin X, et al. Overexpression of NAG-1/GDF15 prevents hepatic steatosis through inhibiting oxidative stress-mediated dsDNA release and AIM2 inflammasome activation. Redox Biol. 2022;52:102322.[DOI]
-
31. Li P, Lv H, Zhang B, Duan R, Zhang X, Lin P, et al. Growth differentiation Factor 15 protects SH-SY5Y cells from rotenone-induced toxicity by suppressing mitochondrial apoptosis. Front Aging Neurosci. 2022;14:869558.[DOI]
-
32. Wang SF, Chang YL, Liu TY, Huang KH, Fang WL, Li AFY, et al. Mitochondrial dysfunction decreases cisplatin sensitivity in gastric cancer cells through upregulation of integrated stress response and mitokine GDF15. FEBS J. 2024;291(6):1131-1150.[DOI]
-
33. Flaherty III SE, Song L, Albuquerque B, Rinaldi A, Piper M, Shanthappa DH, et al. GDF15 neutralization ameliorates muscle atrophy and exercise intolerance in a mouse model of mitochondrial myopathy. J Cachexia Sarcopenia Muscle. 2025;16(1):e13715.[DOI]
-
34. Forsström S, Jackson CB, Carroll CJ, Kuronen M, Pirinen E, Pradhan S, et al. Fibroblast growth factor 21 drives dynamics of local and systemic stress responses in mitochondrial myopathy with mtDNA deletions. Cell Metab. 2019;30(6):1040-1054.[DOI]
-
35. Kim KH, Lee MS. GDF15 as a central mediator for integrated stress response and a promising therapeutic molecule for metabolic disorders and NASH. Biochim Biophys Acta Gen Subj. 2021;1865(3):129834.[DOI]
-
36. Wedel S, Martic I, Guerrero Navarro L, Ploner C, Pierer G, Jansen‐Dürr P, et al. Depletion of growth differentiation factor 15 (GDF15) leads to mitochondrial dysfunction and premature senescence in human dermal fibroblasts. Aging Cell. 2023;22(1):e13752.[DOI]
-
37. Chiariello A, Conte G, Rossetti L, Trofarello L, Salvioli S, Conte , M . Different roles of circulating and intramuscular GDF15 as markers of skeletal muscle health. Front Endocrinol. 2024;15:1404047.[DOI]
-
38. Aguilar-Recarte D, Barroso E, Guma A, Pizarro-Delgado J, Peña L, Ruart M, et al. GDF15 mediates the metabolic effects of PPARβ/δ by activating AMPK. Cell Rep. 2021;36(6):109501.[DOI]
-
39. Campderrós L, Moure R, Cairó M, Gavaldà-Navarro A, Quesada-López T, Cereijo R, et al. Brown adipocytes secrete GDF15 in response to thermogenic activation. Obesity. 2019;27(10):1606-1616.[DOI]
-
40. Romanello V, Sandri M. Implications of mitochondrial fusion and fission in skeletal muscle mass and health. Semin. Cell Dev Biol. 2022;143:46-53.[DOI]
-
41. Kim KH, Kim SH, Han DH, Jo YS, Lee YH, Lee MS. Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Sci Rep. 2018;8(1):6789.[DOI]
-
42. Xiong WP, Yao WQ, Wang B, Liu K. BMSCs-exosomes containing GDF-15 alleviated SH-SY5Y cell injury model of Alzheimer’s disease via AKT/GSK-3β/β-catenin. Brain Res Bull. 2021;177:92-102.[DOI]
-
43. Moon JS, Goeminne LJ, Kim JT, Tian JW, Kim SH, Nga HT, et al. Growth differentiation factor 15 protects against the aging-mediated systemic inflammatory response in humans and mice. Aging Cell. 2020;19(8):e13195.[DOI]
-
44. Fu W, Lai Y, Li K, Yang Y, Guo X, Gong Q, et al. Neurotensin-neurotensin receptor 2 signaling in adipocytes suppresses food intake through regulating ceramide metabolism. Cell Res. 2025;35(2):117-131.[DOI]
-
45. Nevola R, Epifani R, Imbriani S, Tortorella G, Aprea C, Galiero R, et al. GLP-1 receptor agonists in non-alcoholic fatty liver disease: Current evidence and future perspectives. Int J Mol Sci. 2023;24(2):1703.[DOI]
-
46. Zhang Y, Zhao X, Dong X, Zhang Y, Zou H, Jin Y, et al. Activity-balanced GLP-1/GDF15 dual agonist reduces body weight and metabolic disorder in mice and non-human primates. Cell Metab. 2023;35(2):287-298. e4.[DOI]
-
47. Rizvi AA, Patti AM, Giglio RV, Nikolic D, Amato A, Al-Busaidi N, et al. Liraglutide improves carotid intima-media thickness in patients with type 2 diabetes and non-alcoholic fatty liver disease: An 8-month prospective pilot study. Expert Opin Biol Ther. 2015;15(10):1391-1397.[DOI]
-
48. Yang Y, Zhou Y, Wang Y, Wei X, Wang T, Ma A. Exendin-4 regulates endoplasmic reticulum stress to protect endothelial progenitor cells from high-glucose damage. Mol Cell Probes. 2020;51:101527.[DOI]
-
49. Ozcan M, Guo Z, Ripoll CV, Diab A, Picataggi A, Rawnsley D, et al. Sustained alternate-day fasting potentiates doxorubicin cardiotoxicity. Cell Metab. 2023;35(6):928-942.e4.[DOI]
-
50. Kim J, Kim SH, Kang H, Lee S, Park SY, Cho Y, et al. TFEB-GDF15 axis protects against obesity and insulin resistance as a lysosomal stress response. Nat Metab. 2021;3(3):410-427.[DOI]
-
51. L’homme L, Sermikli BP, Haas JT, Fleury S, Quemener S, Guinot V, et al. Adipose tissue macrophage infiltration and hepatocyte stress increase GDF-15 throughout development of obesity to MASH. Nat Commun. 2024;15(1):7173.[DOI]
-
52. Min KW, Liggett JL, Silva G, Wu WW, Wang R, Shen RF, et al. NAG-1/GDF15 accumulates in the nucleus and modulates transcriptional regulation of the Smad pathway. Oncogene. 2016;35(3):377-388.[DOI]
-
53. Osada M, Park HL, Park MJ, Liu JW, Wu G, Trink B, et al. A p53-type response element in the GDF15 promoter confers high specificity for p53 activation. Biochem Biophys Res Commun. 2007;354(4):913-918.[DOI]
-
54. Zhang Y, Hou R, Sun W, Guo J, Chen Z, Li H, et al. Evaluation of large-scale plasma proteomics for prediction of heart failure in individuals with a full range of glucose metabolism profiles. Eur J Prev Cardiol. 2025;zwaf381.[DOI]
-
55. Plantone D, Pardini M, Manco C, Righi D, Alì PA, Arnaldi D, et al. CSF IL-6, GDF-15, GFAP and NfL levels in early Alzheimer disease: a pilot study. Ther Adv Neurol Disord. 2025;18:17562864251314772.[DOI]
-
56. Kamper RS, Nygaard H, Praeger-Jahnsen L, Ekmann A, Ditlev SB, Schultz M, et al. GDF-15 is associated with sarcopenia and frailty in acutely admitted older medical patients. J Cachexia Sarcopenia Muscle.2024;15(4):1549-1557.[DOI]
-
57. Eddy AC, Trask AJ. Growth differentiation factor-15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev. 2021;57:11-18 (2021).[DOI]
-
58. Braig S, Denkinger MD, Dallmeier D, Klenk J, Rothenbacher D, ActiFE Study Group. Social isolation, loneliness and the relationship with serum biomarkers, functional parameters and mortality in older adults. Aging Clin Exp Res. 2025;37(1):140.[DOI]
-
59. Liu CC, Trumpff C, Huang Q, Juster RP, Picard M. Biopsychosocial correlates of resting and stress-reactive salivary GDF15: Preliminary findings. BioRxiv [Preprint]. 2025.[DOI]
-
60. Liu X, Pan S, Xanthakis V, Vasan RS, Psaty BM, Austin TR, et al. Plasma proteomic signature of decline in gait speed and grip strength. Aging Cell. 2022;21(12):e13736.[DOI]
-
61. Lu WH, Guyonnet S, Martinez LO, Lucas A, Parini A, Vellas B, et al. Association between aging-related biomarkers and longitudinal trajectories of intrinsic capacity in older adults. GeroScience. 2023;45(6):3409-3418.[DOI]
-
62. Kiss LZ, Nyárády BB, Pállinger É, Lux Á, Jermendy ÁL, Csobay-Novák C, et al. Association of growth and differentiation factor-15 with coronary artery calcium score and ankle-brachial index in a middle-aged and elderly Caucasian population sample free of manifest cardiovascular disease. GeroScience.2024; 46(1):343-1350.[DOI]
-
63. Conte M, Sevini F, Conte G, Tognocchi M, Ciurca E, Trofarello L, et al. The combination of GDF15, FGF21, sRAGE and NfL plasma levels can identify frailty in community-dwelling people across old age. Mech Ageing Dev. 2025;226:112077.[DOI]
-
64. Fountain WA, Milcik N, Schmedding N, Bandeen-Roche K, Alzahrani MK, Buta B, et al. Baseline plasma GDF15 and recovery of physical function following total knee replacement in the study of physical resilience and aging. J Gerontol A Biol Sci Med Sci. 2025;glaf115.[DOI]
-
65. Oppong R, Orru V, Marongiu M, Qian Y, Sidore C, Delitala A, et al. Age-associated increase in growth differentiation factor 15 levels correlates with central arterial stiffness and predicts all-cause mortality in a Sardinian population cohort. J Am Heart Assoc. 2025;14(10):e036253.[DOI]
-
66. Luo JW, Duan WH, Song L, Yu YQ, Shi DZ. A meta-analysis of growth differentiation factor-15 and prognosis in chronic heart failure. Front Cardiovasc Med. 2021;8:630818.[DOI]
-
67. McGrath , ER , Himali JJ, Levy D, Conner SC, DeCarli C, Pase MP, et al. Growth differentiation factor 15 and NT-proBNP as blood-based markers of vascular brain injury and dementia. J Am Heart Assoc. 2020;9(19):e014659.[DOI]
-
68. Blew CO, Duggan MR, Joynes CM, Gomez GT, Tian Q, Pilling LC, et al. Multi‐cohort analyses link plasma GDF15 with dementia, brain atrophy, and plasma biomarkers. Alzheimers Dement. 2024;20:e086953.[DOI]
-
69. Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020;187:111215.[DOI]
-
70. Falvino A, Bonanni R, Gasperini B, Cariati I, Chiavoghilefu A, Smakaj A, et al. Altered expression of cell cycle regulators and factors released by aged cells in skeletal muscle of patients with bone fragility: A pilot study on the potential role of SIRT1 in muscle atrophy. Biomedicines. 2025;13(6):1350.[DOI]
-
71. Cheng CK, Huang X, Meng S, Huang Y. The pro-aging and rejuvenating effects of young and aged perivascular adipose tissues on endothelial function and inflammation. Biogerontology. 2025;26(4):134.[DOI]
-
72. Xiong W, Li D, Feng Y, Jia C, Zhang X, Liu Z. CircLPAR1 promotes neuroinflammation and oxidative stress in APP/PS1 mice by inhibiting SIRT1/Nrf-2/HO-1 axis through destabilizing GDF-15 mRNA. Mol Neurobiol. 2023;60(4):2236-2251.[DOI]
-
73. Xia M, Zhang Q, Zhang Y, Li R, Zhao T, Chen L, et al. Growth differentiation factor 15 regulates oxidative stress-dependent ferroptosis post spinal cord injury by stabilizing the p62-Keap1-Nrf2 signaling pathway. Front Aging Neurosci. 2022;14:905115 (2022).[DOI]
-
74. Satta S, Beal R, Smith R, Luo X, Ferris GR, Langford-Smith A, et al. A Nrf2-OSGIN1&2-HSP70 axis mediates cigarette smoke-induced endothelial detachment: implications for plaque erosion. Cardiovasc Res. 2023;119(9):1869-1882.[DOI]
-
75. Song H, Chen Q, Xie S, Huang J, Kang G. GDF-15 prevents lipopolysaccharide-mediated acute lung injury via upregulating SIRT1. Biochem Biophys Res Commun. 2020;526(2):439-446.[DOI]
-
76. Chen C, Shi J. GDF-15 alleviates hypoxia-reoxygenation-induced damage to human placental vascular endothelial cells by regulating SIRT1. Cureus. 2024;16(8):e66073.[DOI]
-
77. Conte M, Giuliani C, Chiariello A, Iannuzzi V, Franceschi C, Salvioli , S . GDF15, an emerging key player in human aging. Ageing Res Rev. 2022;75:101569.[DOI]
-
78. Almohaimeed GM, Alonazi AS, Alshammari TK, Dayel AFB, Alghibiwi HK, Alamin MA, et al. Metformin-mediated protection against immunosenescence in diabetic cardiomyopathy: The potential roles of GDF-15 and klotho proteins. Int Immunopharmacol. 2025;153:114530.[DOI]
-
79. Alonazi AS, Aloraini RM, Albulayhi LM, Alshehri LM, Bin Dayel AF, Alamin MA, et al. Macrophage depletion alleviates immunosenescence in diabetic kidney by modulating GDF-15 and Klotho. Int J Mol Sci. 2025;26(9):3900.[DOI]
-
80. Chiariello A, Valente S, Pasquinelli G, Baracca A, Sgarbi G, Solaini G, et al. The expression pattern of GDF15 in human brain changes during aging and in Alzheimer’s disease. Front Aging Neurosci. 2023;14:1058665.[DOI]
-
81. Groarke JD, Crawford J, Collins SM, Lubaczewski SL, Breen DM, Harrington MA, et al. Phase 2 study of the efficacy and safety of ponsegromab in patients with cancer cachexia: PROACC-1 study design. J Cachexia Sarcopenia Muscle. 2024;15(3):1054-1061.[DOI]
-
82. Groarke JD, Crawford J, Collins SM, Lubaczewski S, Roeland EJ, Naito T, et al. Ponsegromab for the treatment of cancer cachexia. N Engl J Med. 2024;391(24):2291-2303.[DOI]
-
83. Lee J, Kim I, Ryu J, Eling T, Baek SJ. NAG-1/GDF15 as a tumor suppressor in colorectal cancer: inhibition of β-catenin and NF-κB pathways via interaction with EpCAM. Cell Death Dis. 2025;16(1):355.[DOI]
-
84. He R, Shi J, Xu D, Yang J, Shen Y, Jiang YS, et al. SULF2 enhances GDF15-SMAD axis to facilitate the initiation and progression of pancreatic cancer. Cancer Lett. 2022;538:215693.[DOI]
-
85. Li L, Zhang R, Yang H, Zhang D, Liu J, Li J, et al. GDF15 knockdown suppresses cervical cancer cell migration in vitro through the TGF‐β/Smad2/3/Snail1 pathway. FEBS Open Bio. 2020;10(12):2750-2760.[DOI]
-
86. Joo M, Kim D, Lee MW, Lee HJ, Kim JM. GDF15 promotes cell growth, migration, and invasion in gastric cancer by inducing STAT3 activation. Int J Mol Sci. 2023;24(3):2925.[DOI]
-
87. Xue W, Li Y, Ma Y, Zhang F. GDF15-mediated enhancement of the Warburg effect sustains multiple myeloma growth via TGFβ signaling pathway. Cancer Metab. 2025;13(1):3.[DOI]
-
88. Huang X, Liang X, Han Q, Shen Y, Chen J, Li Z, et al. Pretreatment with growth differentiation factor 15 augments cardioprotection by mesenchymal stem cells in myocardial infarction by improving their survival. Stem Cell Res Ther. 2024;15(1):412.[DOI]
-
89. Yan B, Zhang B, Chu T, Zhang W, Shi C, Wang H, et al. Osimertinib combined with anlotinib as first-line treatment in advanced or metastatic NSCLC patients with EGFR mutation: a prospective, single arm, exploratory study. Lung Cancer. 2025;205:108627.[DOI]
-
90. Song H, Yin D, Liu Z. GDF-15 promotes angiogenesis through modulating p53/HIF-1α signaling pathway in hypoxic human umbilical vein endothelial cells. Mol Biol Rep. 2011;39(4):4017-4022.[DOI]
-
91. Park H, Nam KS, Lee HJ, Kim KS. Ionizing radiation-induced GDF15 promotes angiogenesis in human glioblastoma models by promoting VEGFA expression through p-MAPK1/SP1 signaling. Front Oncol. 2022;12:801230.[DOI]
-
92. Dong G, Zheng QD, Ma M, Wu SF, Zhang R, Yao RR, et al. Angiogenesis enhanced by treatment damage to hepatocellular carcinoma through the release of GDF 15. Cancer Med. 2018;7(3):820-830.[DOI]
-
93. Qin H, Chen J, Bouchekioua-Bouzaghou K, Meng YM, Griera JB, Jiang X, et al. Immunization with a multi-antigen targeted DNA vaccine eliminates chemoresistant pancreatic cancer by disrupting tumor-stromal cell crosstalk. J Transl Med. 2023;21(1):702.[DOI]
-
94. Modi A, Purohit P, Roy D, Vishnoi JR, Pareek P, Elhence P, et al. FOXM1 mediates GDF-15 dependent stemness and intrinsic drug resistance in breast cancer. Mol Biol Rep. 2022;49(4):2877-2888.[DOI]
-
95. Bellio C, Emperador M, Castellano P, Gris-Oliver A, Canals F, Sánchez-Pla A, et al. GDF15 is an eribulin response biomarker also required for survival of DTP breast cancer cells. Cancers. 2022;14(10):2562.[DOI]
-
96. Lin H, Luo Y, Gong T, Fang H, Li H, Ye G, et al. GDF15 induces chemoresistance to oxaliplatin by forming a reciprocal feedback loop with Nrf2 to maintain redox homeostasis in colorectal cancer. Cell Oncol. 2024;47(4):1149-1165.[DOI]
-
97. Du Y, Ma Y, Zhu Q, Fu Y, Li Y, Zhang Y, et al. GDF15 negatively regulates chemosensitivity via TGFBR2-AKT pathway-dependent metabolism in esophageal squamous cell carcinoma. Front Med. 2022;17(1):119-131.[DOI]
-
98. Huang Fu Z, Xiao M, Xie H, Zhang S, Yi T, Li Q, et al. Suppressing GDF15 enhances the chemotherapeutic effect of 5 FU on MSI-H CRC by regulating the ferroptosis pathway SLC7A11/GSH/GPX4. J Cancer Res Clin Oncol. 2024;151(1):6.[DOI]
-
99. Lu Q, Liao Y. GDF-15 upregulates the SLC7A11/GPX4 signaling axis and promotes mitoxantrone resistance in AML cells. Eur J Med Res. 2025;30(1):504.[DOI]
-
100. Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U.S.A. 2011;108(50):20012-20017.[DOI]
-
101. Flores RR, Clauson CL, Cho J, Lee BC, McGowan SJ, Baker DJ, et al. Expansion of myeloid-derived suppressor cells with aging in the bone marrow of mice through a NF-kB-dependent mechanism. Aging Cell. 2017;16(3):480-487.[DOI]
-
102. Onorati A, Havas AP, Lin B, Rajagopal J, Sen P, Adams PD, et al. Upregulation of PD-L1 in senescence and aging. Mol Cell Biol. 2022;42(10):e00171-22.[DOI]
-
103. Wang TW, Johmura Y, Suzuki N, Omori S, Migita T, Yamaguchi K, et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature. 2022;611(7935):358-364.[DOI]
-
104. Dong Z, Luo Y, Yuan Z, Tian Y, Jin T, Xu F. Cellular senescence and SASP in tumor progression and therapeutic opportunities. Mol Cancer. 2024;23(1):181.[DOI]
-
105. Cimino I, Kim H, Tung YCL, Pedersen K, Rimmington D, Tadross JA, et al. Activation of the hypothalamic-pituitary-adrenal axis by exogenous and endogenous GDF15. Proc Natl Acad Sci U.S.A. 2021;118(27):e2106868118.[DOI]
-
106. Segerer SE, Rieger L, Kapp M, Dombrowski Y, Müller N, Dietl J, et al. MIC-1 (a multifunctional modulator of dendritic cell phenotype and function) is produced by decidual stromal cells and trophoblasts. Hum Reprod. 2011;27(1):200-209.[DOI]
-
107. Wang Z, He L, Li W, Xu C, Zhang J, Wang D, et al. GDF15 induces immunosuppression via CD48 on regulatory T cells in hepatocellular carcinoma. J Immunother Cancer. 2021;9(9):e002787.[DOI]
-
108. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057-1061.[DOI]
-
109. Zhou Z, Li W, Song Y, Wang L, Zhang K, Yang J, et al. Growth differentiation factor-15 suppresses maturation and function of dendritic cells and inhibits tumor-specific immune response. PLoS One. 2013;8(11):e78618.[DOI]
-
110. Gao Y, Xu Y, Zhao S, Qian L, Song T, Zheng J, et al. Growth differentiation factor-15 promotes immune escape of ovarian cancer via targeting CD44 in dendritic cells. Exp Cell Res. 2021;402(1):112522.[DOI]
-
111. Chen S, Huang C, Liao G, Sun H, Xie Y, Liao C, et al. Distinct single-cell immune ecosystems distinguish true and de novo HBV-related hepatocellular carcinoma recurrences. Gut. 2023;72(6):1196-1210.[DOI]
-
112. Zheng H, Yu S, Zhu C, Guo T, Liu F, Xu Y. HIF1α promotes tumor chemoresistance via recruiting GDF15-producing TAMs in colorectal cancer. Exp Cell Res. 2021;398(2):112394.[DOI]
-
113. Ratnam NM, Peterson JM, Talbert EE, Ladner KJ, Rajasekera PV, Schmidt CR, et al. NF-κB regulates GDF-15 to suppress macrophage surveillance during early tumor development. J Clin Invest. 2017;127(10):3796-3809.[DOI]
-
114. Si Y, Liu X, Cheng M, Wang M, Gong Q, Yang Y, et al. Growth differentiation factor 15 is induced by hepatitis C virus infection and regulates hepatocellular carcinoma-related genes. PLoS One. 2011;6(5):e19967.[DOI]
-
115. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565-1570.[DOI]
-
116. Jung SB, Choi MJ, Ryu D, Yi HS, Lee SE, Chang JY, et al. Reduced oxidative capacity in macrophages results in systemic insulin resistance. Nat Commun. 2018;9(1):1551.[DOI]
-
117. Urakawa N, Utsunomiya S, Nishio M, Shigeoka M, Takase N, Arai N, et al. GDF15 derived from both tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression via Akt and Erk pathways. Lab Invest. 2015;95(5):491-503.[DOI]
-
118. Xie H, Zhang K, Yin H, Zhang S, Pan S, Wu R, et al. Acetyltransferase NAT10 inhibits T-cell immunity and promotes nasopharyngeal carcinoma progression through DDX5/HMGB1 axis. J Immunother Cancer. 2025;13(2):e010301..[DOI]
-
119. Han B, He J, Chen Q, Yuan M, Zeng X, Li Y, et al. ELFN1-AS1 promotes GDF15-mediated immune escape of colorectal cancer from NK cells by facilitating GCN5 and SND1 association. Discov Onc. 2023;14(1):56.[DOI]
-
120. Roth P, Junker M, Tritschler I, Mittelbronn M, Dombrowski Y, Breit SN, et al. GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin Cancer Res. 2010;16(15):3851-3859.[DOI]
-
121. Haake M, Haack B, Schäfer T, Harter PN, Mattavelli G, Eiring P, et al. Tumor-derived GDF-15 blocks LFA-1 dependent T cell recruitment and suppresses responses to anti-PD-1 treatment. Nat Commun. 2023;14(1):4253.[DOI]
-
122. Benichou O, Coskun T, Gonciarz MD, Garhyan P, Adams AC, Du Y, et al. Discovery, development, and clinical proof of mechanism of LY3463251, a long-acting GDF15 receptor agonist. Cell Metab. 2023;35(2):274-286.[DOI]
-
123. Melero I, de Miguel Luken M, de Velasco G, Garralda E, Martín-Liberal J, Joerger M, et al. Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumours. Nature. 2025;637(8048):1218-1227.[DOI]
-
124. Carneiro BA, Gbolahan OB, Abdul Razak AA, Hilton JF, Lambert AW, Hood J, et al. First-in-human study to evaluate the safety and efficacy of anti-GDF15 antibody AZD8853 in patients with advanced/metastatic solid tumors. Cancer Res Commun. 2025;5(6):896-905.[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




