Dongdong Xia, Department of Orthopaedic Surgery, The First Affiliated Hospital of Ningbo University, 59 Liuting Street, Haishu District, Ningbo 315010, Zhejiang, China. E-mail: drdongdongxia@163.com
Abstract
The effectiveness of hemostasis during spinal surgery directly influences the surgical success rate and patient prognosis. Minimally invasive surgery has multiple advantages, including reductions in intraoperative and postoperative blood loss, minimized damage to surrounding tissues, and alleviation of postoperative pain. However, when the spinal epidural venous plexus is located within deep cavities, achieving sufficient hemostasis often presents challenges due to limited operating space and the limitations of traditional hemostatic materials. In recent years, novel adhesive hemostatic hydrogels have served as local injectable hemostatic agents. These hydrogels function through dual mechanisms: physical blockage of bleeding sites and biological activation, such as promoting coagulation cascades, thereby showing significant advantages in bleeding control at complex anatomical sites. Accordingly, this article systematically reviews the progress of basic research and clinical translation related to hemostatic materials over the past five years to provide a scientific foundation for optimizing perioperative hemostatic strategies.
Keywords
1. Introduction
In the surgical field, effective intraoperative hemostasis is fundamental to ensuring procedural safety and optimizing postoperative recovery. Spinal surgery, involving critical structures such as the spinal cord, nerve roots, and epidural venous plexus, presents unique challenges due to its complex anatomy, limited surgical field, and concealed bleeding sites. These factors demand exceptional precision and adaptability from both hemostatic techniques and materials. Hemostatic materials are available in a wide range of forms[1]. For instance, film and powder materials, such as SURGICELTM, can be applied directly to relatively flat bleeding sites in open surgery. Additionally, powder formulations (e.g., AristaTM) can be applied through endoscopic delivery. Sponge materials, primarily composed of gelatin (e.g., SurgifoamTM, GelfoamTM) or collagen (e.g., AviteneTM), feature a porous structure that facilitates hemostasis in minor wounds through physical expansion and the provision of a matrix to support platelet aggregation. However, with the ongoing evolution of spinal surgery toward minimally invasive procedures (such as endoscopic techniques and percutaneous interbody fusion) and highly complex operations (such as spinal tumor resection and severe deformity correction), these conventional forms of hemostatic materials have shown increasing limitations. Their poor adaptability to irregular deep bleeding and insufficient tissue conformity often lead to postoperative hematoma or tissue adhesion. In contrast, gel- or paste-based materials (e.g., SurgifloTM, FlosealTM) exhibit fluidity, allowing precise injection through narrow surgical channels into deep or irregular spaces, achieving intimate contact with complex bleeding surfaces. These properties make them particularly advantageous in minimally invasive spine surgery.
Hemostasis relies on the finely coordinated interplay of vasoconstriction, platelet activation and aggregation, and the coagulation cascade. Good hemostatic materials should exhibit biocompatibility, controllable degradability, rapid onset of hemostatic activity, and no interference with tissue healing[2,3]. As the foundational components of many modern hemostatic systems, natural polymers provide essential biochemical and structural features that have directly informed the design of next-generation adhesive hydrogels for spinal surgery. Natural polymeric materials boast excellent biocompatibility and biodegradability. Their degradation products are non-toxic, and they exhibit good absorption of the materials in vivo, as well as low immunogenicity. Moreover, natural polymers contain active groups, such as amino groups and carboxyl groups, that promote hemostasis by adsorbing red blood cells and activating coagulation pathways. They also enhance platelet adhesion and aggregation. In contrast, synthetic polymers are primarily crosslinked polymers (e.g., polyethylene glycol, polyacrylamide), which are often blended with natural materials to balance biocompatibility and mechanical stability, providing more versatile solutions for complex bleeding scenarios in spinal surgery. Synthetic polymers further expand these foundational concepts by enabling tunable mechanical strength, controlled degradation, and programmable chemical functionalities, properties that have become central to the development of advanced adhesive hemostatic hydrogels. Taken together, the progression from natural to synthetic polymer systems has laid the technological and mechanistic foundation for the emergence of adhesive hemostatic hydrogels, which integrate bioactivity, mechanical sealing, and tissue-specific adhesion to meet the complex bleeding challenges of spinal surgery. Hydrogels, as injectable 3D crosslinked networks of natural or synthetic polymers, have been extensively reviewed by Veglianese et al. for applications in spinal cord injury, highlighting their capacity for in situ delivery of bioactive molecules or cells while maintaining biocompatibility, tunable mechanical properties, and controlled degradation[4].
Based on these considerations, this review systematically summarizes the composition, hemostatic mechanisms, and research progress of natural polymer-based hemostatic materials (e.g., cellulose-, starch-, and chitosan-based), synthetic polymer-based materials (e.g., polyethylene glycol (PEG)- and polyacrylamide (PAAm)-based), and composite hydrogels (Figure 1). It further highlights the clinical applications and evidence of commonly used flowable hemostatic products, such as FlosealTM and SurgifloTM (gelatin–thrombin matrices) and HemofenceTM (hyaluronic acid (HA)-based), in spinal surgeries including lumbar canal decompression, lumbar corpectomy with fusion, and spinal tumor resection. It should be noted that most polymeric hydrogel hemostats remain at the preclinical stage. Their safety (e.g., prevention of cerebrospinal fluid leakage and thrombosis), applicability (e.g., compatibility with endoscopic precision delivery), and long-term metabolic safety in spinal procedures still lack robust clinical evidence and warrant further investigation. This article aims to summarize recent progress and highlight key issues for future clinical translation.
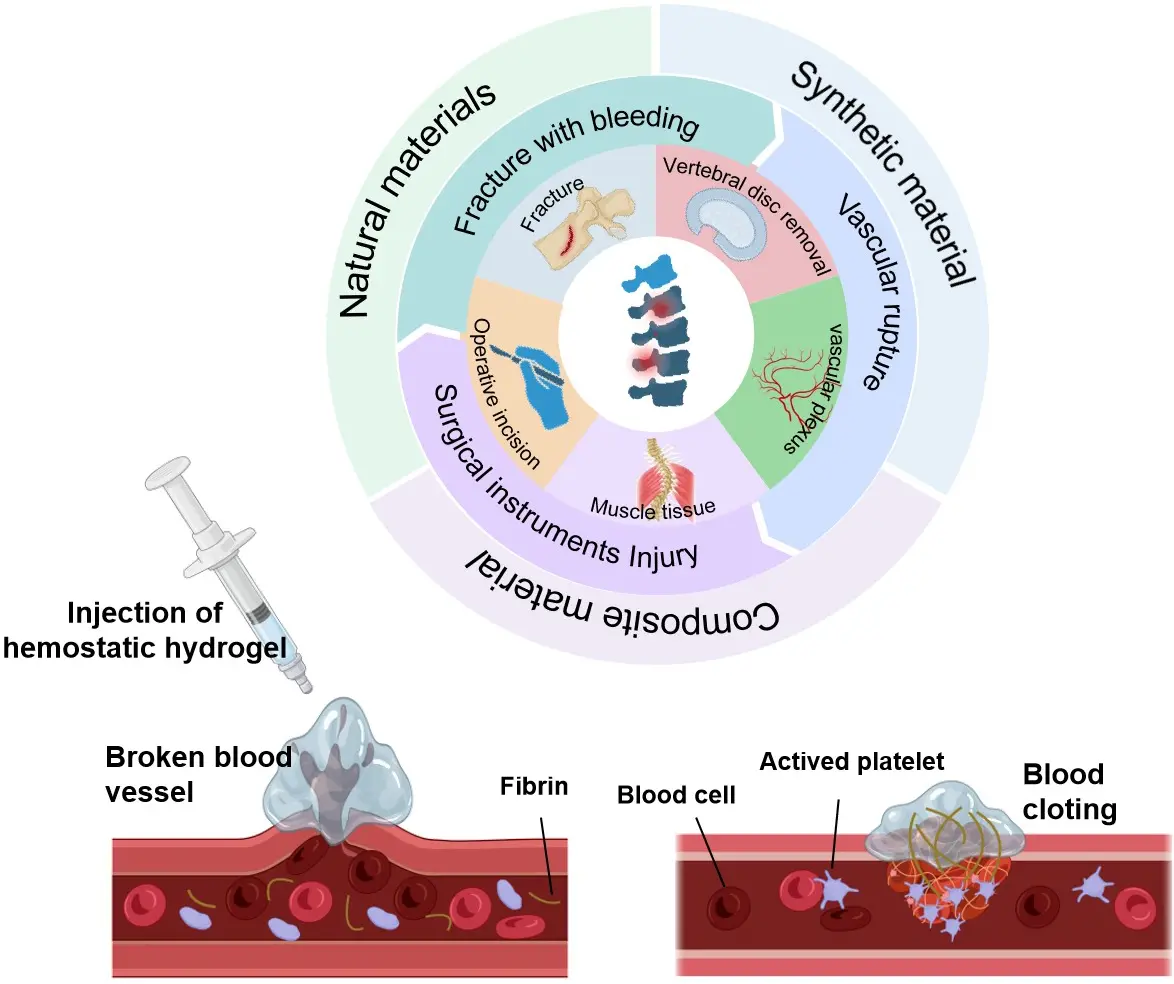
Figure 1. A variety of natural, synthetic polymers and composite materials utilized to engineer hemostatic hydrogels. Created in BioRender.com.
Figure 1 summarizes the primary sources of intraoperative bleeding encountered in spinal procedures, the typical anatomical sites where bleeding arises, and the corresponding mechanistic targets for hemostatic intervention. Specifically, bleeding in spinal surgery can originate from (1) epidural venous plexuses (low-pressure venous oozing), where materials that provide rapid adhesion and conformal sealing are required; (2) cancellous bone surfaces (e.g., during decompression or fusion), where absorptive matrices and procoagulant factors that concentrate platelets are advantageous; (3) arterial or arteriolar injuries, where rapid thrombin-mediated coagulation is essential; and (4) tumor-related friable vasculature, which may demand combined mechanical occlusion and local procoagulant activation. By mapping these bleeding sources to their optimal material functions (physical occlusion, platelet activation, ionic release, enzymatic catalysis, or adhesive sealing), Figure 1 provides the conceptual framework used throughout this review to assess material suitability in specific surgical scenarios. This mapping is discussed in more detail in the sections that follow.
2. Natural Polymer Materials
2.1 Cellulose-base
Cellulose is widely sourced from plants, bacteria, and algae; it exhibits hydrophilicity, biocompatibility, and a low-cost advantage. Common cellulose-derived gauzes, such as oxidized regenerated cellulose (ORC) and sodium carboxymethyl cellulose (CMC), have been extensively used for hemostasis in surgical procedures. Additionally, cellulose-based hydrogels are still under development[5]. Guan et al. developed a CMC-collagen XVII hydrogel achieving rapid hemostasis in a rat liver model (sealing time 99.3 s, blood loss 259 mg)[6]. Deng reported cellulose-flaxseed gum hydrogels with high porosity and swelling[7], and Liu et al. developed IPN hydrogels with cellulose nanofibers, gelatin, and Ag nanoparticles showing both hemostatic and antibacterial properties[8]. Zhang et al. fabricated bacterial cellulose hydrogels with glucose oxidase-integrated metal-organic framework nanocatalysts, achieving rapid hemostasis and antibacterial effects[9]. Overall, cellulose-based hydrogels combine rapid hemostasis with potential antibacterial activity, but their mechanical weakness and enzymatic susceptibility suggest that composite designs are necessary for clinical translation.
2.2 Starch-base
Starch, a typical plant-derived polysaccharide, has glucose polymer chains containing numerous closely spaced reactive hydroxyl (-OH) groups, which give it strong water-binding capacity. Currently, the representative commercial powdered hemostat AristaTM AH is made by reacting purified potato starch with epichlorohydrin to form microporous polysaccharide microspheres[10]. Upon contact with blood, these microspheres rapidly absorb water and low-molecular-weight compounds via a “molecular sieve” effect. This process concentrates platelets and coagulation proteins, which then aggregate on the microspheres to form fibrin clots. However, starch-based powders exhibit poor adhesion to tissue interfaces and have limited ability to penetrate deeply into bleeding cavities. Therefore, researchers have begun to focus on starch-based hydrogels. Zheng et al.[11] constructed an oxidized starch hydrogel loaded with calcium ions (Ca2+) using a green hot-extrusion 3D- printing technique, which demonstrated effective acute hemostasis and promoted skin wound healing. Cui et al.[12] developed an enzyme-cross-linked hydrogel based on dopamine-modified starch (St-Dopa), which exhibited excellent tissue adhesion and rapid gelation. In an in vivo rabbit liver injury model, the St-Dopa hydrogel significantly shortened hemostatic time and reduced blood loss. These studies demonstrate that hydrogel-based starch materials can overcome the limitations of powdered forms, achieving faster hemostasis and enhanced tissue compatibility.
2.3 Chitosan-based
Chitosan is the only natural occurring cationic polysaccharide that contains amino groups. Under acidic conditions, the -NH2 groups in chitosan protonate to form -NH3+ groups. This protonation enhances platelet adhesion and aggregation and promotes blood clot formation by interacting with negatively charged platelets and red blood cells[13]. Various modifications have been developed to enhance its hemostatic performance. For example, Janus self-propelled chitosan microspheres[14], hydroxybutyl chitosan-dopamine hydrogels[15,16], and quaternized or tannic acid (TA)-crosslinked derivatives[17-21] exhibit rapid gelation, strong adhesion, and antibacterial properties. Clinically, LifeGelTM, a hydrophobic chitosan-based hydrogel, is under investigation for use in spinal surgery[21]. Overall, these studies illustrate how chemical modification and composite design can tailor chitosan-based hydrogels for efficient and multifunctional hemostasis.
2.4 Alginate-based
Alginate, an anionic linear polysaccharide, forms a Ca2+-crosslinked three-dimensional gel network that physically traps red blood cells, activates platelets, and sustains the coagulation[22]. Jiang et al. reported a platelet-based hydrogel composed of alginate and silver nanoparticles (AgNPs) showing significant hemostatic efficacy and promoting wound healing in a mouse liver hemorrhage model[22]. Zhai et al. synthesized a cell-adhesive peptide-alginate hydrogel (Pept-1/ALG/Ca2+) achieving rapid coagulation in vitro (41 s) and markedly reduced blood loss in vivo[23]. Alginate-based hydrogels effectively trigger coagulation and support wound healing, but their mechanical properties and degradation behavior may require optimization for broader clinical application.
2.5 HA-based
HA hydrogels can be chemically modified to enhance adhesion, swelling, and drug delivery. Mussel-inspired dopamine conjugation and other functionalizations have enabled rapid hemostasis and anti-inflammatory activity[24-27]. For example, DMHA hydrogels achieved hemostasis within 12.2 s in a rat liver injury model and demonstrated high adhesive strength[24]. pH-responsive HA hydrogels (HA@F127-MF) allowed sustained drug release while preventing bleeding[25]. Oxidized/aminated HA hydrogels (OxAy series) showed excellent injectability and efficient intraoperative hemostasis during endoscopic submucosal dissection[26]. HA-based hydrogels provide tunable adhesion, rapid gelation, and multifunctional hemostatic effects, making them promising candidates for intraoperative applications.
2.6 Gelatin-based
Gelatin hydrogels leverage the coagulation-mimicking properties of collagen and can be modified via crosslinking or incorporation of bioactive components. Visible-light-crosslinked Gel-SH/GelMA/PD-LAP hydrogels reduced blood clotting time to 1.5 min[27,28]. GelMA/γPGA-NHS systems showed strong adhesion and effective hemostasis in rat and porcine models[29]. Bioadhesives incorporating snake venom hemocoagulase achieved rapid clot formation through a dual mechanism, physical barrier combined with enzymatic catalysis[30]. Gelatin-based hydrogels offer versatile strategies to enhance hemostasis through both physical and enzymatic mechanisms, with potential for translational application in surgery.
2.7 Small molecule
Platelet-rich plasma, an autologous blood component, promotes wound healing and hemostasis through thrombin generation and fibrin polymerization[31,32]. Short-chain polyphosphate released from activated platelets serves as a key regulator by accelerating coagulation factor V activation and enhancing clot formation[32]. Natural polyphenols such as TA and gallic acid, rich in catechol and pyrocatechol groups, can bind blood components to promote platelet aggregation and coagulation factor activation, achieving rapid wound sealing and prevention of secondary bleeding[33]. Traditional Chinese medicine ingredients, including Panax notoginseng[34], Bletilla striata polysaccharides[35], and aloe polysaccharide extracts[36], have also demonstrated hemostatic potential. Peptide-based small molecules provide additional strategies for hemostasis. RADA16, a synthetic amphiphilic 16-amino-acid peptide, self-assembles into a 3D hydrogel under physiological conditions, exhibiting low immunogenicity and good biocompatibility. In sheep endoscopic nasal surgery, RADA16 achieved intraoperative hemostasis comparable to gelatin-thrombin, reduced postoperative adhesions, and promoted tissue regeneration[37,38]. Similarly, h9e peptide hydrogel, derived from spider silk and human muscle sequences, demonstrated rapid hemostasis (94 s in rats) and effective wound sealing[39].
Small molecule and peptide-based materials complement natural polymers by providing rapid hemostasis, bioactivity, and injectable hydrogel formation. While retaining advantages such as anti-inflammatory and regenerative functions, these materials share limitations, including insufficient mechanical strength and enzymatic susceptibility, highlighting the need for composite or chemically modified designs to optimize performance for clinical application[40] (Table 1).
| Base materials | Source | Core Composition | Hemostasis mechanism | Additional function | Refs. |
| Cellulose-based | Plants (cotton, wood), bacteria | CMC–recombinant type XVII collagen; bacterial cellulose–glucose oxidase@MOF nanocatalysts; ORC/CMC | 1. Physical occlusion (absorbs fluid to concentrate coagulation components); 2. Activates platelets/coagulation factors | Antibacterial properties; promotes wound healing (repair function) | [5,6,9] |
| Starch-based | Plants (potato, corn) | Potato starch–epichlorohydrin (microporous microspheres); oxidized starch–Ca2+; St-Dopa–enzyme cross-linking system | 1. “Molecular sieve effect” of microspheres (absorbs fluid to concentrate platelets/coagulation proteins); 2. Physical sealing of wounds via hydrogel | Promotes wound healing | [10-12] |
| Chitosan-based | Deacetylation of chitin | Chitosan–CaCO3–protonated tranexamic acid (TXA-NH3+); QCS–TA; HBC–dopamine; LifeGelTM hydrophobic chitosan–fatty acids–inert reagents | 1. Cations (-NH3+) interact with negatively charged red blood cells/platelets to promote aggregation; 2. Physical occlusion; 3. Enzyme-catalyzed coagulation | Antibacterial properties; low-swelling design | [13,14,18,21] |
| Alginate-based | Brown algae | Sodium alginate–natural platelets–AgNPs; sodium alginate–calcium chloride (CaCl2)–Pept-1 | 1. Ca2+-crosslinked hydrogel network encapsulates red blood cells/activates platelets; 2. Sustained release of Ca2+ triggers coagulation cascade | Antibacterial properties; promotes wound healing; tissue adhesiveness | [22,23] |
| HA-based | Natural glycosaminoglycans | DMHA; OHA–aminated HA (HA-ADH)–MF; HA–PL 407 | 1. High swelling property enables physical hemostasis; 2. Dopamine-mediated tissue adhesion; 3. Sustained drug release aids hemostasis | Anti-inflammatory properties; sustained drug release; promotes wound healing | [24-27] |
| Gelatin-based | Animal collagen (acid/alkali hydrolysis) | Gel-SH–GelMA–PD-LAP; GelMA–N-hydroxysuccinimide-modified poly-γ-glutamic acid (γPGA-NHS); GelMA–Bothrops atrox thrombin | 1. Physical barrier of hydrogel; 2. Snake venom thrombin catalyzes conversion of fibrinogen to fibrin; 3. Activates coagulation factors | Promotes wound healing | [28-30] |
| Small-molecule Natural Materials | Autologous blood, plants, synthetic peptides | PRP; tannic acid–CMC; TCP-25; RADA16 peptide; h9e peptide (spider silk elastic segment–human muscle transmembrane segment) | 1. PRP releases polyphosphates to activate coagulation FV; 2. Polyphenols promote platelet aggregation; 3. Peptide self-assembly forms 3D physical barrier | Anti-inflammatory properties; promotes mucosal repair; low immunogenicity | [31-39] |
| PEG-based | Chemical synthesis | Four-armed PEG hydrogel (TetraStat); succinate-structured four-armed PEG hydrogel; dual-syringe in-situ forming Tetra-PEG hydrogel | 1. pH-responsive rapid solidification forms physical barrier; 2. Coagulation-independent physical sealing of wounds | In-situ rapid gelation via dual-syringe system (~6 seconds); stable hydrogel network structure; tunable physicochemical properties; easy functional modification; controllable degradation; low-swelling design | [41-43] |
| PAAm-based | Chemical synthesis | PAAm-TA-KA; MHA-PAAm–gelatin–AgNPs | 1. KA activates coagulation factor XII to accelerate coagulation cascade; 2. TA provides adhesiveness + physical cross-linking to enhance mechanical properties; 3. Physical barrier for hemostasis | High mechanical stability; excellent adhesiveness on wet wound surfaces; scalable production | [44,45] |
| PVA-based | Chemical synthesis | 15% PVA aqueous solution | 1. Physical barrier for wound compression; 2. High water absorption capacity accelerates coagulation factor concentration | Good injectability; excellent compressive deformation resistance and viscoelasticity; physicochemical properties tunable by adjusting cross-linking degree | [46] |
| PU-based | Chemical synthesis | PU hydrogel containing Schiff base cross-linker and catechol chain extender | 1. pH-responsive physical barrier for wound sealing; 2. Catechol activates platelets and coagulation factors for hemostasis | Excellent elasticity and mechanical toughness; self-healing property; antibacterial and anti-inflammatory effects; promotes wound healing | [47] |
CMC: carboxymethyl cellulose; MOF: metal-organic framework; ORC: oxidized regenerated cellulose; QCS: quaternized chitosan; PAAm-TA-KA: polyacrylamide-tannic acid-kaolin; HBC: hydroxybutyl chitosan; NP: nanoparticle; DMHA: dopamine-conjugated maleic hyaluronic acid; OHA: oxidized hyaluronic acid; HA-ADH: aminated hyaluronic acid; MF: mometasone furoate; PL 407: poloxamer 407; Gel-SH: thiolated gelatin; GelMA: gelatin methacrylate; PD-LAP: polydopamine functionalized Laponite®; PRP: platelet-rich plasma; FV: factor V; PEG: polyethylene glycol; MHA: methacrylated hyaluronan; PVA: polyvinyl alcohol; PU: polyurethane; St-Dopa: dopamine-modified starch; Pept-1: cell-adhesive peptide; AgNPs: silver nanoparticles; NHS: N-hydroxysuccinimide.
3. Synthetic-Based Materials
PEG is a water-soluble polymer that can undergo controllable degradation via chemical functionalization. Shinya Okata[41] reported a tetra-PEG hydrogel, TetraStat, which rapidly solidifies in response to pH changes. In a rat inferior vena cava puncture model, it achieved hemostasis within 1 minute with only mild inflammation. He et al.[42] developed a succinyl-ester-structured four-armed PEG hydrogel that enabled coagulation-independent hemostasis, stopping bleeding within 30 seconds and resulting in 38 mg of blood loss in a heparinized rat tooth extraction model, outperforming clinical cotton and gelatin sponges. Bu et al.[43] reported an injectable Tetra-PEG hydrogel that gelled in situ within approximately 6 seconds using a dual-syringe system, demonstrating excellent hemostatic performance in a porcine massive splenic hemorrhage model, even under anticoagulation. The hydrogel degraded completely within 5 days, and its degradation products were eliminated via renal excretion.
PAAm is a linear polymer with high water solubility and interfacial adhesion, but it is not biodegradable. Fan et al.[44] fabricated polyacrylamide-tannic acid-kaolin hybrid hydrogels, in which TA provided adhesive catechol groups and kaolin acted as both a physical crosslinker and a contact activator of coagulation factor FXII. Activation of FXII accelerated fibrin formation and thrombus generation, shortening hemostatic time to 24-31 s in a rat femoral artery hemorrhage model, far faster than the blank control (148 s). Tang et al.[45] developed methacrylated hyaluronan–polyacrylamide hydrogels combined with gelatin and AgNPs to enhance adhesion and antibacterial activity. In rat tail amputation and liver bleeding models, these adhesives reduced hemostatic time to 16.8 ± 3.6 s and 30.5 ± 4.3 s, and blood loss to 203 mg and 0.14 ± 0.08 g, respectively, outperforming both the no-treatment and gauze groups. In addition, they promoted healing and reduced inflammation in acute wound infection models. Polyvinyl alcohol (PVA)[46] and polyurethane (PU)[47] have also been explored to develop injectable hydrogels with hemostatic function for controlling bleeding. Chemical modification of PEG and PVA derivatives may introduce trace toxic monomers, while PU- and PAAm-based materials can generate harmful degradation products and exhibit slow in vivo degradation. Although short-term hemostatic safety is generally controllable, long-term metabolic toxicity requires further investigation.
4. Composite Hydrogel
4.1 Natural components
The combination of various natural components is a common strategy for developing hemostatic materials. Yang et al.[48] developed a collagen-starch (CoSt)-based hydrogel. This hydrogel demonstrates high toughness, strong and long-lasting adhesion on wet surfaces, and excellent hemostatic performance. In multiple non-compressible hemostasis models, such as rat tail amputation, liver incision, abdominal aortic incision, and complete transection of the spinal cord causing hemorrhage, it was able to control massive bleeding within 60 seconds.
As shown in Figure 2, the hemostatic performance and mechanism of CoSt hydrogels were systematically evaluated in a rat spinal cord transection model. Figure 2A schematically illustrates the establishment of the transected spinal cord injury and corresponding bleeding model. Figure 2B presents the incompressible in situ hemostatic mechanism of the CoSt hydrogel, which rapidly adheres to irregular wound surfaces and forms a stable barrier to block blood flow. Figure 2C shows the limited hemostatic effect of a commercial gelatin sponge, while Figure 2D demonstrates the superior efficacy of the CoSt hydrogel under the same conditions[48]. These results further confirm the strong in situ hemostatic capability of the CoSt system.
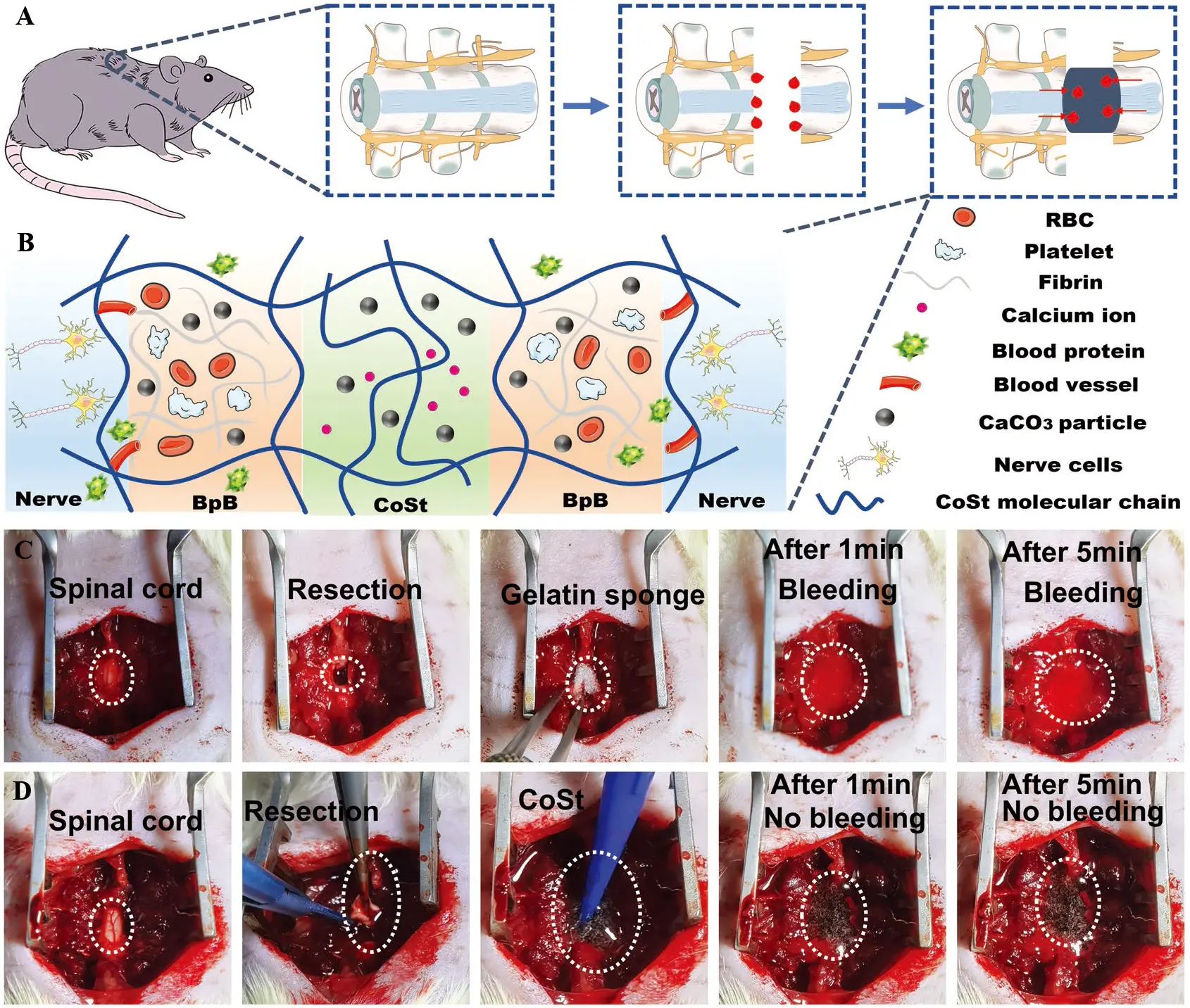
Figure 2. The hemostatic perfomance and mechanism of CoSt hydrogels were systematically evaluated in a rat spinal cord transection model. (A) Schematic illustration of the transected spinal cord injury bleeding of the rat and hemostatic model; (B) Schematic illustrations of the incompressible in situ hemostatic mechanism of CoSt hydrogels; (C) Hemostasis test in rat transected nerve injury model of commercial gelatin sponge; (D) CoSt hydrogels. Republished with permission from[48]. RBC: red blood cell; BpB: blood protein barrier; CoSt: collagen-starch.
Zou et al.[49] developed multifunctional bioadhesive hydrogels comprising modified carboxymethyl chitosan, modified sodium alginate, and modified TA. They exhibited an adhesive strength of 162.6 ± 7.0 kPa, which was notably higher than the adhesive strength of commercial fibrin glue (13.2 ± 4.9 kPa). In the rabbit liver injury model, they caused less blood loss (0.32 ± 0.10 g) compared with the fibrin glue group (0.59 ± 0.05 g). Furthermore, these hydrogels had antibacterial and antioxidant properties and were applicable for first-aid hemostasis and the healing of infected wounds. Chen et al.[50] reported an injectable superstructure consisting of thrombin-loaded nanorobots incorporated into regenerated silk fibroin nanofibril hydrogels. This superstructure is designed for percutaneous injection into spinal metastatic lesions derived from hemorrhagic hepatocellular carcinoma. Driven by the photothermal effect generated by gold (Au) nanorods within the nanorobots, thrombin is released in a controlled manner, efficiently inducing thrombosis, blocking tumor blood vessels, and achieving hemostasis.
4.2 Natural polymers using synthetic materials
In addition, the modification and optimization of natural polymers using synthetic materials is also an important strategy. Ren et al.[51] prepared hydrogel adhesives by mixing HA derivatives (HA-ADH and HA-DTPH) with 4aPEG-OPA through a catalyst-free OPA/N-nucleophile condensation reaction. In rat liver and rabbit femoral artery-vein hemorrhage models, the hydrogel enabled rapid hemostasis and achieved better wound healing than fibrin glue or cyanoacrylate. As illustrated in Figure 3, the formulation, mechanism, and applications of multifunctional hydrogel adhesives are summarized. Figure 3A presents the biomimetic CoSt hydrogel developed by Yang et al.[48], highlighting its formulation, structural stability, and in situ hemostatic performance. Figure 3B illustrates the multifunctional hydrogel designed by Zou et al.[49] for first-aid hemostasis and infected wound healing, integrating antibacterial and antioxidant properties. Figure 3C depicts the fabrication of hydrogel adhesives through mixing HA-ADH or HA-DTPH with 4aPEG-OPA, forming a catalyst-free OPA/N-nucleophile condensation network. Figure 3D shows the dynamic cross-linking mechanism responsible for both the bulk hydrogel structure and firm hydrogel–tissue adhesion. Figure 3E demonstrates the application of these hydrogels in hemostatic sealing and skin wound closure, achieving rapid bleeding control and enhanced tissue repair[51]. Together, these studies emphasize the versatility of hydrogel-based systems in combining mechanical strength, bioadhesion, and biological functionality for diverse clinical hemostatic applications.
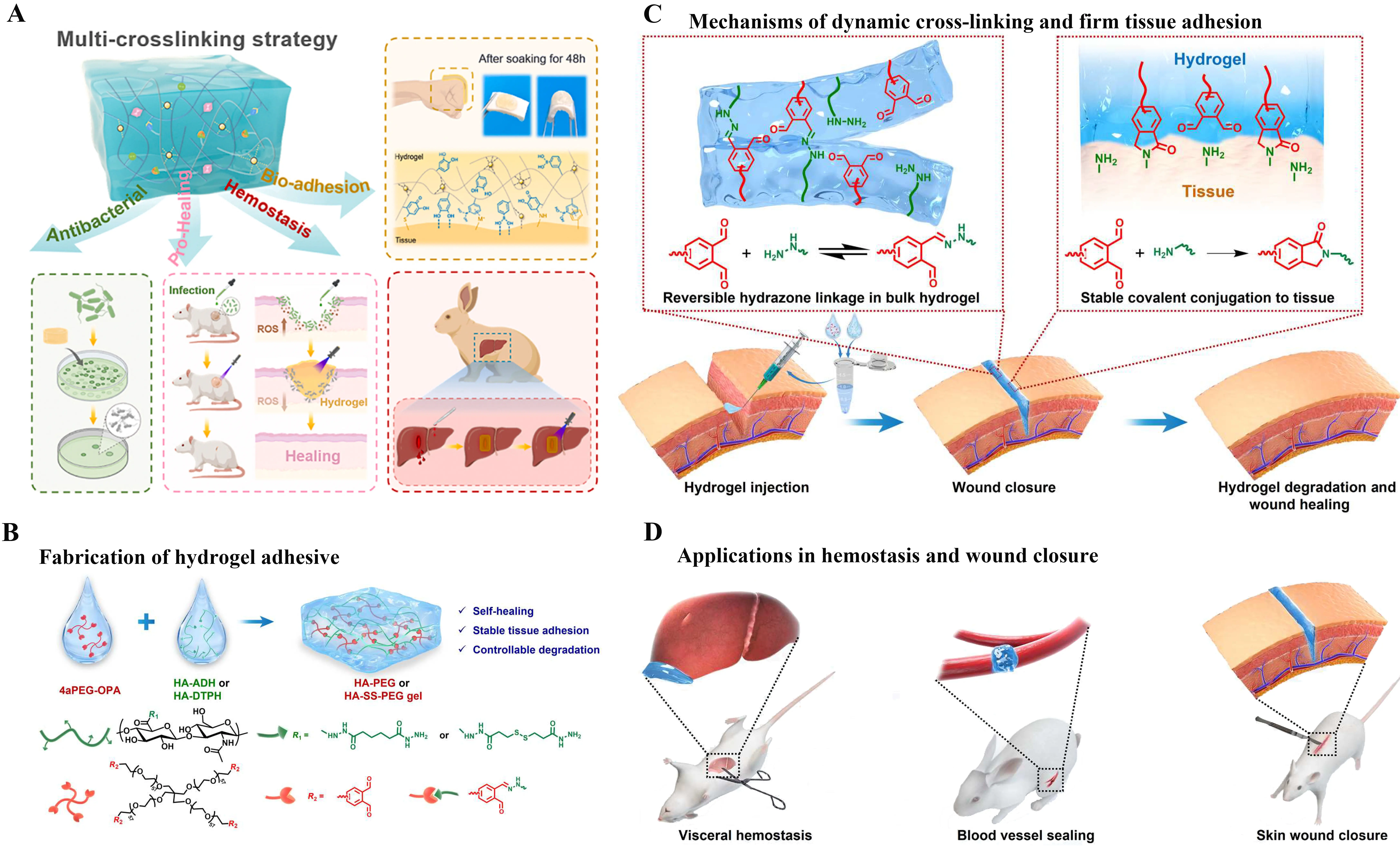
Figure 3. The fabrication, mechanisms, and applications of multifunctional hydrogel adhesives. (A) A multifunctional hydrogel was constructed by integrating three biocompatible components: CMCS as the structural matrix, SA for ionic crosslinking, and TA functioning as both a biological crosslinker and an active therapeutic agent. The hydrogel exhibits robust bio-adhesion and pro-coagulant activity, key properties that drive excellent in vivo hemostatic performance. Additionally, the hydrogel demonstrates potent inhibitory effects against E. coli (gram-negative) and S. aureus (gram-positive), effectively suppressing bacterial proliferation at the wound site and thereby facilitating the healing of infected wounds[49]; (B) Hydrogel adhesives are fabricated by mixing HA-ADH or disulfide-containing hydrazide-modified HA (HA-DTPH) with 4aPEG-OPA, leveraging the OPA/N-nucleophile condensation reaction as the crosslinking mechanism; (C) The hydrogels exhibit rapid, firm adhesion to various tissues via an established OPA-mediated mechanism, holding great potential for sutureless wound closure and hemostatic sealing; (D) In liver and blood vessel injuries, the hydrogels effectively seal incisions and rapidly achieve hemostasis. In rat models of full-thickness skin incisions, they also quickly close wounds and accelerate healing[51]. CMCS: carboxymethyl chitosan; SA: sodium alginate; TA: tannic acid; HA-ADH: aminated hyaluronic acid; OPA: o-phthalaldehyde; 4aPEG-OPA: o-phthalaldehyde-terminated four-armed poly(ethylene glycol).
Zheng et al.[52] prepared a hemostatic hydrogel with components including N-hydroxysuccinimide (NHS)-conjugated alginate, poly(ethylene glycol) diacrylate, TA, and Fe3+. Dual adhesive moieties (pyrogallol/catechol and NHS) synergistically enhanced wet tissue adhesion, while TA/Fe3+ boosted hemostasis via physical sealing and high blood affinity. Additionally, Li et al.[53] used N-succinyl chitosan (NSC) and oxidized HA (OHA) as precursors, and incorporated Ca2+ and a polyethylene glycol derivative (PEG1) to modulate the cross-linking and mechanical properties. In a mouse liver hemorrhage model, Ca2+-containing NSC-OHA hydrogels showed excellent hemostatic effects, reducing blood loss.
5. Hemostatic Hydrogel in Spinal Surgery
Over the past 20 years, both the number and complexity of spinal surgeries have risen. Inadequate intraoperative bleeding control can lead to a range of adverse outcomes. Endoscopic techniques have gained widespread recognition for treating spinal disorders due to their advantages of minimal bleeding, reduced trauma, and shorter operation times[54]. However, traditional non-flowable hemostatic materials are limited. Recently developed hemostatic hydrogels address these limitations. They can be injected through catheters into narrow, irregular spaces. This allows them to adhere directly to bleeding sites in various scenarios, such as diffuse oozing, hard-to-reach bleeding locations, and cases requiring preventive hemostasis, which have quickly become a focus of current research.
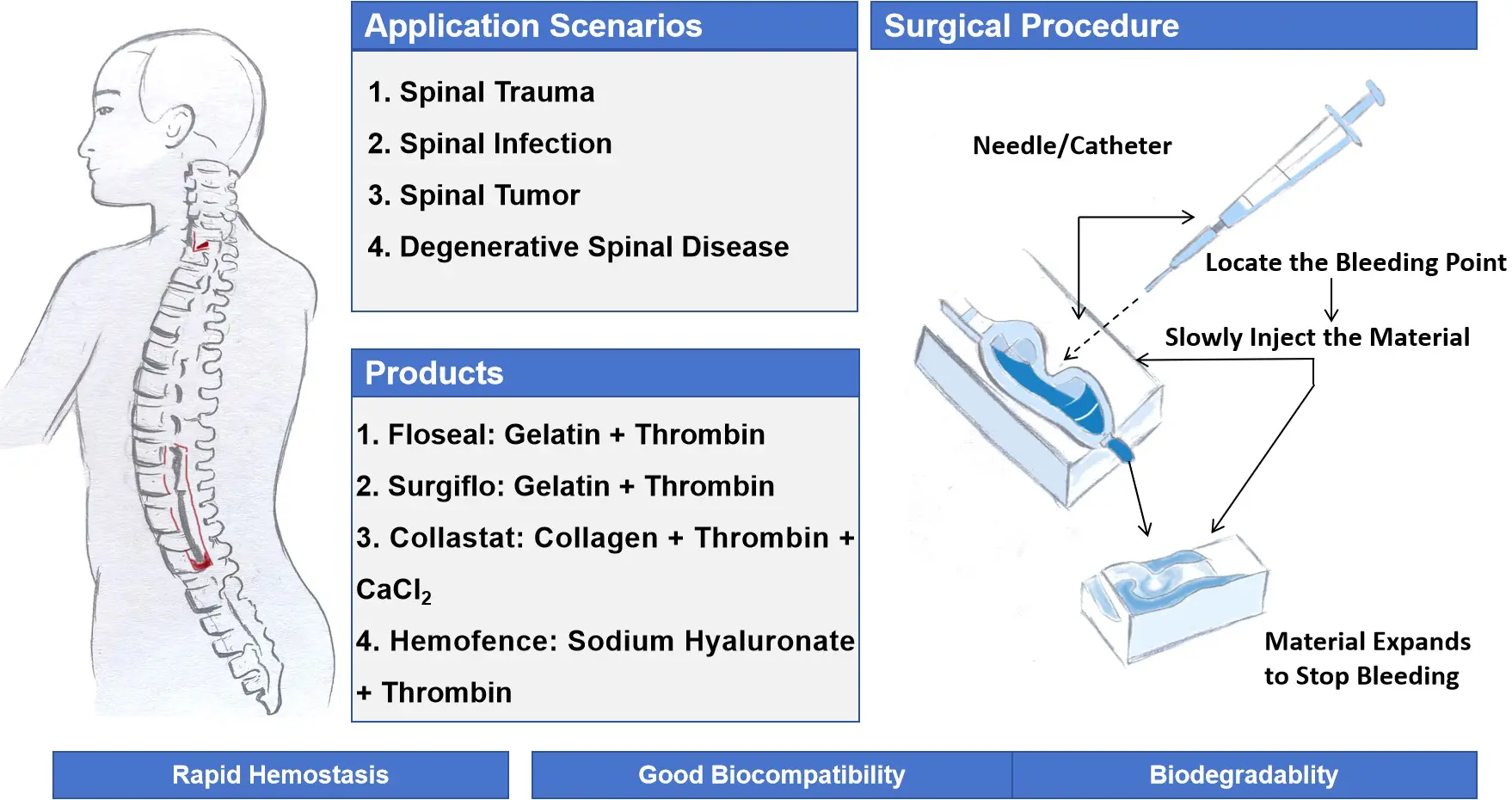
As illustrated in Figure 4, different in situ forming hemostatic hydrogels are applied in spinal surgery. The two panels on the left present the major clinical application scenarios, including spinal trauma, spinal infection, spinal tumor, and degenerative spinal diseases, together with representative commercial products currently available, such as Floseal® (gelatin + thrombin), Surgiflo® (gelatin + thrombin), Collastat® (collagen + thrombin + CaCl2), and Hemofence® (sodium hyaluronate + thrombin). These products share the advantages of rapid hemostasis, good biocompatibility, anti-adhesion properties, and biodegradability. The panel on the right schematically illustrates the surgical procedure of in situ hydrogel injection: after identifying the bleeding point with a needle or catheter, the hemostatic hydrogel is slowly injected into the target site, where it expands to form a physical barrier that effectively stops bleeding.
Collectively, Figure 4 demonstrates the diverse applications and mechanisms of in situ forming hemostatic hydrogels in spinal surgery, highlighting their translational potential in managing intraoperative bleeding.
5.1 Floseal®
Floseal® consists of gelatin and thrombin and is widely used for hemostasis in various spinal surgeries, including those for spinal tumors, degenerative spinal disorders, and spinal trauma[55]. In microscopic endoscopic decompression surgeries for lumbar spinal stenosis in elderly male patients[56], results showed that Floseal® not only reduced bleeding and lowered the risk of spinal epidural hematoma but also helped improve postoperative neurological function recovery. Postoperative physical examinations and preliminary MRI evaluations of patients revealed no signs of infection, confirming the agent’s efficacy and safety. Similarly, in single-level spinal stenosis decompression surgery using the biportal endoscopic spine surgery technique, Floseal® achieved hemostasis within 3 minutes. Furthermore, it reduced the postoperative hematoma incidence rate by 50% compared with standard treatment[57]. Notably, postoperative residual Floseal® particles are considered foreign bodies that may trigger inflammatory reactions and granulomatous responses, which may subsequently lead to radicular pain[58]. Therefore, saline irrigation should be initiated promptly and continued after surgery to remove hematomas and residual Floseal® particles.
5.2 Surgiflo®
Surgiflo®, another thrombin-gelatin matrix hemostatic agent, demonstrates excellent hemostatic efficacy and biocompatibility. In a rat laminectomy model, compared with the control group (laminectomy alone and no hemostatic agent) and other conventional hemostatic materials (including ORC [Pahacel®], polysaccharide hemostats [Sealfoam®], and chitosan-based agents [CeloxTM]), Surgiflo® achieved the lowest epidural fibrosis (EF) grade (mean grade: 0.75) and significantly reduced the severity of EF (p < 0.001)[59]. Posterior lumbar interbody fusion is used to treat conditions such as lumbar instability, intervertebral space collapse, and severe spinal stenosis, and is now regarded as one of the most challenging procedures in minimally invasive spine surgery. In an observational study[60], the hemostatic efficacy of Surgiflo® was evaluated in single-level transforaminal lumbar interbody fusion. A total of 102 patients were enrolled, with 54 assigned to the Surgiflo® group and 48 to the control group, where hemostasis was achieved solely via bipolar electrocoagulation and gauze compression. Intraoperatively, Surgiflo® was injected at bleeding sites (e.g., near nerve roots and the epidural venous plexus), and the complex of Surgiflo® and blood clots was irrigated and removed two minutes after injection. Data showed that compared with the control group, the Surgiflo® group had a ~29.3% reduction in intraoperative blood loss, a ~13.6% shorter surgical time, and an ~11.2% shorter hospital stay. MRI assessments at 1 week postoperatively revealed a ~17.4% smaller hematoma size in the Surgiflo® group. Correlation analysis demonstrated a negative correlation between Surgiflo® dosage and intraoperative blood loss (r = -0.313, p = 0.002). Multivariate regression analysis further confirmed that Surgiflo® dosage (standardized coefficient: -0.220, p = 0.032) was an independent factor influencing intraoperative blood loss. Patients with lumbar spinal stenosis face heightened risk, as long-term compression of the epidural venous plexus thins venous vessel walls, increasing the likelihood of intraoperative spinal canal bleeding[61,62]. In patients undergoing lumbar endoscopic unilateral laminotomy for bilateral decompression, flowable gelatin (liquid SHM) was injected endoscopically at epidural venous plexus bleeding sites. Compared with the control group, the SHM group showed a significant increase (≥ 20%) in the 3-minute hemostasis success rate. It rapidly controlled epidural venous plexus bleeding without repeated compression or electrocoagulation, reducing surgical field interference. Additionally, the SHM group had shorter surgical duration and significantly less perioperative blood loss (including intraoperative and 48-hour postoperative bleeding). No adverse events, such as epidural hematoma, allergic reactions, or thrombosis, were observed, thus avoiding neurological dysfunction caused by hematoma compression[63].
5.3 Other fluid hemostatic agents
CollaStat is a thrombin-containing collagen-based hemostatic agent with a paste-like, flowable consistency. Its formulation consists of two connectable syringes: one prefilled with porcine skin-derived telopeptide-free collagen and thrombin, and the other containing a CaCl2 solution. When mixed, the two components are ready for immediate use and can achieve hemostasis within 1 minute. In a study by Park et al.[64], the hemostatic efficacy and safety of CollaStat and Floseal were compared in 18 patients undergoing spinal tumor resection and reconstruction, 9 in the CollaStat group and 9 in the Floseal group. Results showed that the hemostasis success rates were 88.89% (8/9) in the CollaStat group and 100% (9/9) in the Floseal group, with a mean hemostasis time of 1 minute and a hemostatic agent dosage of 1 unit per patient in both groups. No statistically significant differences were observed between the two groups in terms of postoperative 3-day drainage volume (CollaStat: 406.3 ± 213.6 mL vs. Floseal: 326.6 ± 169.5 mL), hospital stay duration (CollaStat: 8.33 ± 3.12 days vs. Floseal: 7.44 ± 2.60 days), or adverse event rate (CollaStat: 22.22% vs. Floseal: 33.33%) (all p > 0.05). These findings confirm that CollaStat is non-inferior to Floseal in terms of hemostatic efficacy and safety. Sungjae An and colleagues conducted a multicenter[65], randomized phase III clinical trial to evaluate the hemostatic efficacy and safety of Hemofence, a novel hemostatic agent, for refractory oozing bleeding in spinal surgery. The study used Floseal (a thrombin-gelatin matrix), a commonly used clinical agent, as the control. Hemofence’s core components consist of thrombin and sodium hyaluronate, which form a stable gel matrix through a crosslinking reaction. Thrombin, a key component that initiates the coagulation process, effectively promotes blood clotting to achieve hemostasis. Sodium hyaluronate, on the other hand, acts as a backbone for the gel matrix: it not only provides reliable carrier support for thrombin but also endows the material with excellent biocompatibility and tissue adaptability. Results from the clinical trial showed that Hemofence achieved a hemostatic success rate of 97.10% within 10 minutes. Compared with the control agent Floseal (96.05%), Hemofence showed no statistically significant difference in hemostatic time. Additionally, there were no statistically significant differences in the incidence of adverse events, adverse drug reactions, or serious adverse events between Hemofence and the control. These findings confirm that Hemofence represents a safe and effective new option to meet hemostatic needs in spinal surgery.
6. Clinical Selection of Hemostatic Materials
To provide clinicians with more practical guidance, the selection of hemostatic hydrogels can be considered from three operative perspectives: the nature of bleeding, the surgical environment, and the condition of the target tissue. First, different bleeding patterns place distinct demands on hydrogel performance. Capillary or diffuse oozing favors materials with high wettability, rapid in situ formation, and strong interfacial adhesion. Venous bleeding typically requires hydrogels that balance adhesion with adequate mechanical cohesion, while high-pressure arterial bleeding may necessitate reinforced systems capable of fast cross-linking and resisting significant burst pressures.
Second, the surgical setting also influences material suitability. In open procedures, viscous or bulk-forming hydrogels are generally acceptable. In contrast, minimally invasive or endoscopic operations require low-swelling, injectable, and fast-curing formulations that can be precisely delivered through narrow working channels. For anatomically confined regions such as the spine or skull base, hydrogels with minimal expansion and good mechanical stability help reduce the risk of postoperative compression. Finally, tissue characteristics should be taken into account. Fragile organs such as the liver or spleen benefit from softer, highly adhesive hydrogels that minimize additional trauma. When tissue regeneration is desirable, bioactive or biodegradable hydrogels may provide complementary therapeutic benefits. Viewed together, these considerations form a practical, decision-oriented framework for matching specific hydrogel properties to real surgical scenarios (Table 2).
| Parameter | Subcategory | Recommended Hydrogel Characteristics | Representative Examples | Key Performance Parameters |
| Bleeding type | Capillary/diffuse ooze | High wettability, rapid in situ formation, strong adhesion | Catechol-modified hydrogels, Schiff-base hydrogels | Gelation time ≤ 30 s; Adhesion strength ≥ 20 kPa; Minimal swelling |
| Venous/moderate bleeding | Balanced adhesion and moderate mechanical strength | HA-based bulk hydrogels | Burst pressure ~50-100 mmHg; Moderate stiffness (G' ~1-10 kPa) | |
| Arterial/high-pressure bleeding | Reinforced, rapid cross-linking, high burst pressure | PEG-reinforced hydrogels | Burst pressure ≥ 200 mmHg; Gelation time ≤ 10 s; High toughness | |
| Surgical environment | Open surgery | Viscous or bulk-forming hydrogels acceptable | Standard HA/PEG hydrogels | Easy handling; Moderate swelling; Stiffness G' ~5-15 kPa |
| Minimally invasive/endoscopic | Low-swelling, injectable, fast-curing | Injectable Schiff-base hydrogels | Injectable via 18-22 G; Gelation ≤ 30 s; Low swelling ratio < 20% | |
| Anatomically confined regions (spine, skull base) | Minimal expansion, high mechanical stability | Reinforced, low-swelling hydrogels | Swelling < 10%; G' > 10 kPa; High compressive strength | |
| Tissue condition | Fragile tissues (liver, spleen) | Soft, highly adhesive, minimally traumatic | Catechol-modified soft hydrogels | Low modulus (0.5-5 kPa); Adhesion > 15 kPa |
| Tissues requiring regeneration | Bioactive or biodegradable | Bioactive HA-based hydrogels, degradable PEG hydrogels | Degradation 1-4 weeks; Supports cell proliferation |
HA: hyaluronic acid; PEG: polyethylene glycol.
7. Challenge and Outlook
Hemostatic hydrogels represent an emerging class of materials for minimally invasive spinal surgery, enabling surgeons to maintain a clearer visual field and thereby reducing complications such as nerve root injury and dural hematoma. Despite these advantages, existing commercial hemostatic products, including Huaruo, Bornflow, Kening, and Nashi, have notable limitations. Moreover, most hydrogel-based hemostatic materials remain at the preclinical stage, with insufficient clinical data to support widespread use. Their translation into clinical practice still faces multiple challenges, with three major technical bottlenecks requiring urgent attention.
First, safety concerns must be addressed: hydrogels may infiltrate veins or cerebrospinal fluid, potentially triggering thrombosis or allergic reactions. Second, key technologies such as injection molding, in-situ phase transition, and dynamic adhesion require further optimization to ensure reliable performance. Third, material behavior in vivo poses risks: hydrogels tend to swell upon contact with water, which may compress neural tissues, while adhesive components carry the potential for undesired tissue adhesion. Therefore, future research must prioritize not only hemostatic efficiency but also comprehensive safety evaluation. It is important to note that this review focuses specifically on hemostatic hydrogels for minimally invasive spinal procedures, including endoscopic techniques and interbody fusion. For open surgeries with high blood loss, traditional materials, such as bone wax, hemostatic sponges, and cottonoids, may remain more suitable. Additionally, the use of any hemostatic material should complement rather than replace fundamental surgical techniques. Clinical decision-making should incorporate patient-specific factors such as coagulation status and expected blood loss, with careful management of excess material to avoid complications.
Looking forward, we propose two major directions for advancing hemostatic hydrogel research. On one hand, researchers can refine material design strategies to develop multimodal, functionally integrated hydrogels that combine hemostatic, antibacterial, anti-inflammatory, and tissue-regenerative properties, thereby addressing both bleeding control and microenvironmental modulation. Recent advances from established biomaterials research groups further highlight the feasibility of these directions. For example, work from Reis and the 3B’s Research Group has demonstrated how natural-based hydrogels can be engineered through modular crosslinking strategies to achieve biocompatibility, controlled degradation, and multifunctional bioactivity, principles highly relevant to the design of next-generation hemostatic materials[66]. Furthermore, insights from polymer nanocomposite research by Seifalian’s group also provide valuable guidance for future hydrogel development. Their work on functionalized graphene oxide–poly(carbonate-urea)urethane nanocomposites, which achieve enhanced mechanical stability, tunable elasticity, and improved interfacial properties, demonstrates how nanoscale reinforcement strategies can be leveraged to design safer and more robust injectable biomaterials[67]. Such principles are relevant for advancing hemostatic hydrogels toward improved structural integrity, controlled adhesion, and reduced risk of in vivo deformation or swelling. These advances offer useful frameworks for developing spinal-surgery-specific hemostatic hydrogels with improved stability, controllable adhesion, and adaptive functionality.
On the other hand, the integration of smart technologies, including fluorescent probes, nanocomposites, and magnetic targeting, can enable hydrogels to respond to external stimuli such as pH, temperature, or ionic changes. This responsiveness allows reversible adhesion and targeted hemostasis in specific tissue environments. We believe that addressing these challenges will be critical to safely and effectively translating hemostatic hydrogels from preclinical studies to patient care.
Authors contribution
Gao Q: Writing-original draft, writing-review & editing, data curation.
Zhang C: Writing-original draft, writing-review & editing, supervision, resources, data curation.
Xu R: Resources.
Shi Y: Methodology.
Luo Y: Resources, data curation.
Yu B: Conceptualization.
Xia D: Supervision, funding acquisition.
Conflicts of interest
Yang Luo is a Youth Editorial Board Member of BME Horizon. The other authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (No. LBY24H180003), Ningbo Key Projects of Science and Technology (No. 2023Z192), Traditional Chinese Medicine Science and Technology Program of Zhejiang Province (2024ZL894), and the Natural Science Foundation of Ningbo City (No. 2023J140).
Copyright
© The Author(s) 2025.
References
-
1. Sucandy I, Ross S, DeLong J, Tran M, Qafiti F, Pechman D, et al. TAVAC: Comprehensive review of currently available hemostatic products as adjunct to surgical hemostasis. Surg Endosc. 2024;38(5):2331-2343.
-
2. Sung YK, Lee DR, Chung DJ. Advances in the development of hemostatic biomaterials for medical application. Biomater Res. 2021;25(1):37.
-
3. Brown KGM, Solomon MJ. Topical haemostatic agents in surgery. Br J Surg. 2024;111(1):znad361.
-
4. Pinelli F, Pizzetti F, Veneruso V, Petillo E, Raghunath M, Perale G, et al. Biomaterial-mediated factor delivery for spinal cord injury treatment. Biomedicines. 2022;10(7):1673.
-
5. Chen C, Xi Y, Weng Y. Recent advances in cellulose-based hydrogels for tissue engineering applications. Polymers. 2022;14(16):3335.
-
6. Guan D, Li W, Zhao S, Tao Z, Liu K, Liu Q, et al. Carboxymethyl cellulose-enhanced recombinant human collagen hydrogel promotes hemostasis and wound healing. Int J Biol Macromol. 2025;315:144458.
-
7. Deng Y, Chen J, Huang J, Yang X, Zhang X, Yuan S, et al. Preparation and characterization of cellulose/flaxseed gum composite hydrogel and its hemostatic and wound healing functions evaluation. Cellulose. 2020;27(7):3971-3988.[DOI]
-
8. Liu R, Dai L, Si C, Zeng Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr Polym. 2018;195:63-70.
-
9. Zhang S, Ding F, Liu Y, Ren X. Glucose-responsive biomimetic nanoreactor in bacterial cellulose hydrogel for antibacterial and hemostatic therapies. Carbohydr Polym. 2022;292:119615.
-
10. LyBarger KS. Review of evidence supporting the aristaTM absorbable powder hemostat. Med Devices (Auckl). 2024;173-188.
-
11. Zheng B, Qiu Z, Xu J, Zeng X, Liu K, Chen L. 3D printing-mediated microporous starch hydrogels for wound hemostasis. J Mater Chem B. 2023;11(35):8411-8421.
-
12. Cui R, Chen F, Zhao Y, Huang W, Liu C. A novel injectable starch-based tissue adhesive for hemostasis. J Mater Chem B. 2020;8(36):8282-8293.
-
13. Basa S, Nampally M, Honorato T, Das SN, Podile AR, El Gueddari NE, et al. The pattern of acetylation defines the priming activity of chitosan tetramers. J Am Chem Soc. 2020;142(4):1975-1986.
-
14. Yu Q, Su B, Zhao W, Zhao C. Janus self-propelled chitosan-based hydrogel spheres for rapid bleeding control. Adv Sci. 2023;10(5):e2205989.
-
15. Zhang X, Sun GH, Tian MP, Wang YN, Qu CC, Cheng XJ, et al. Mussel-inspired antibacterial polydopamine/chitosan/temperature-responsive hydrogels for rapid hemostasis. Int J Biol Macromol. 2019;138:321-333.
-
16. Liu S, Ju R, Zhang Z, Jiang Z, Cui J, Liu W, et al. Temperature-sensitive injectable chitosan-based hydrogel for endoscopic submucosal dissection. Int J Biol Macromol. 2024;282:136566.
-
17. Sacco P, Cok M, Scognamiglio F, Pizzolitto C, Vecchies F, Marfoglia A, et al. Glycosylated-chitosan derivatives: A systematic review. Molecules. 2020;25(7):1534.
-
18. Guo S, Ren Y, Chang R, He Y, Zhang D, Guan F, et al. Injectable self-healing adhesive chitosan hydrogel with antioxidative, antibacterial, and hemostatic activities for rapid hemostasis and skin wound healing. ACS Appl Mater Interfaces. 2022;14(30):34455-34469.
-
19. Liao X, Feng X, Xiao Z, Williams GR, Huang X, Shi Y, et al. Multifunctional phenylboric acid modified carboxymethyl chitosan based hydrogel crosslinked by tannic acid. Int J Biol Macromol. 2025;304:140958.
-
20. Wen N, Li S, Jiang H, Yang J, Yang W, Song Y, et al. Bio-inspired self-healing hydrogel for fast hemostasis and accelerated wound healing of gastric ulcers. Adv Funct Mater. 2025;35(1):2411959.[DOI]
-
21. Fang Y, Guo W, Ni P, Liu H. Recent research advances in polysaccharide-based hemostatic materials: A review. Int J Biol Macromol. 2024;271:132559.
-
22. Jiang Y, Wang J, Zhang H, Chen G, Zhao Y. Bio-inspired natural platelet hydrogels for wound healing. Sci Bull. 2022;67(17):1776-1784.
-
23. Zhai Z, Xu K, Mei L, Wu C, Liu J, Liu Z, et al. Co-assembled supramolecular hydrogels of cell adhesive peptide and alginate for rapid hemostasis and efficacious wound healing. Soft Matter. 2019;15(42):8603-8610.
-
24. Fan P, Dong Q, Yang J, Chen Y, Yang H, Gu S, et al. Flexible dual-functionalized hyaluronic acid hydrogel adhesives formed in situ for rapid hemostasis. Carbohydr Polym. 2023;313:120854.
-
25. Meng J, Wang W, Wang J, Xu M. Fabrication and properties of an injectable hyaluronic acid hydrogel loaded with corticosteroid. Eur J Pharm Sci. 2025;211:107096.
-
26. Qin G, Wu R, Wang Q, Sun M, Li Y, Duan S, et al. Injectable hyaluronic acid-based hydrogels for rapid endoscopic submucosal dissection. ACS Biomater Sci Eng. 2024;10(12):7657-7666.
-
27. Varela-Rey I, de la Iglesia D, San Bruno-Ruz A, Mejuto-Fernández R, Monteserín-Ron L, López-Diaz J, et al. Design and biopharmaceutical preclinical characterisation of a new thermosensitive hydrogel for the removal of gastric polyps. Int J Pharm. 2023;635:122706.
-
28. Rajabi N, Kharaziha M, Emadi R, Zarrabi A, Mokhtari H, Salehi S. An adhesive and injectable nanocomposite hydrogel of thiolated gelatin/gelatin methacrylate/Laponite® as a potential surgical sealant. J Colloid Interface Sci. 2020;564:155-169.
-
29. Zhu Z, Ye H, Zhang K, He G, Pan Z, Xian Y, et al. Naturally derived injectable dual-cross-linked adhesive hydrogel for acute hemorrhage control and wound healing. Biomacromolecules. 2024;25(4):2574-2586.
-
30. Guo Y, Wang Y, Zhao X, Li X, Wang Q, Zhong W, et al. Snake extract-laden hemostatic bioadhesive gel cross-linked by visible light. Sci Adv. 2021;7(29)
-
31. Oneto P, Etulain J. PRP in wound healing applications. Platelets. 2021;32(2):189-199.
-
32. Chen R, Huang M, Xu P. Polyphosphate as an antithrombotic target and hemostatic agent. J Mater Chem B. 2023;11(33):7855-7872.
-
33. Bai S, Zhang X, Cai P, Huang X, Huang Y, Liu R, et al. A silk-based sealant with tough adhesion for instant hemostasis of bleeding tissues. Nanoscale Horiz. 2019;4(6):1333-1341.[DOI]
-
34. Wang D, Lv L, Xu Y, Jiang K, Chen F, Qian J, et al. Cardioprotection of Panax Notoginseng saponins against acute myocardial infarction and heart failure through inducing autophagy. Biomed Pharmacother. 2021;136:111287.
-
35. Tang Z, Dan N, Chen Y. Utilizing epoxy Bletilla striata polysaccharide collagen sponge for hemostatic care and wound healing. Int J Biol Macromol. 2024;259:128389.
-
36. Li W, Wang J, Cheng Z, Yang G, Zhao C, Gao F, et al. Sandwich structure Aloin-PVP/Aloin-PVP-PLA/PLA as a wound dressing to accelerate wound healing. RSC Adv. 2022;12(42):27300-27308.
-
37. Lee MF, Ananda A. Self-assembling RADA16 peptide hydrogel supports hemostasis, synechiae reduction, and wound healing in a sheep model of endoscopic nasal surgery. Auris Nasus Larynx. 2023;50(3):365-373.
-
38. Hur K, Adili A, Tam B, Herrera K, Agarwal A, Rice D, et al. Efficacy of a RADA-16 peptide hydrogel versus chitosan-based polymer in improving patient comfort during postoperative debridement: A randomized controlled trial. Int Forum Allergy Rhinol. 2024;14(10):1590-1597.
-
39. Carter T, Qi G, Wang W, Nguyen A, Cheng N, Ju YM, et al. Self-assembling peptide solution accelerates hemostasis. Adv Wound Care. 2021;10(4):191-203.
-
40. Xu C, Xu P, Gao Y, Gao F, Zhuang X, Zhang H, et al. Hierarchically cross-linked Gelatin/Tannic acid/Laponite hybrid antimicrobial hydrogel for hemostatic dressings. Compos Commun. 2023;43:101743.[DOI]
-
41. Okata S, Hoshina K, Hanada K, Kamata H, Fujisawa A, Yoshikawa Y, et al. Hemostatic capability of a novel tetra-polyethylene glycol hydrogel. Ann Vasc Surg. 2022;84:398-404.
-
42. He G, Xian Y, Lin H, Yu C, Chen L, Chen Z, et al. An injectable and coagulation-independent Tetra-PEG hydrogel bioadhesive for post-extraction hemostasis and alveolar bone regeneration. Bioact Mater. 2024;37:106-118.
-
43. Bu Y, Zhang L, Sun G, Sun F, Liu J, Yang F, et al. Tetra-PEG based hydrogel sealants for in vivo visceral hemostasis. Adv Mater. 2019;31(28):e1901580.
-
44. Fan X, Wang S, Fang Y, Li P, Zhou W, Wang Z, et al. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater Sci Eng C Mater Biol Appl. 2020;109:110649.
-
45. Tang Q, Chen C, Jiang Y, Huang J, Liu Y, Nthumba PM, et al. Engineering an adhesive based on photosensitive polymer hydrogels and silver nanoparticles for wound healing. J Mater Chem B. 2020;8(26):5756-5764.
-
46. Dorkhani E, Faryabi A, Noorafkan Y, Heirani A, Behboudi B, Fazeli MS, et al. Biomedical properties and hemostatic efficacy of polyvinyl alcohol (PVA) based hydrogel in experimental rat liver injury model. J Appl Biomater Funct Mater. 2023;21:22808000231198803.
-
47. Li S, Lv H, Yang X. A pH-triggered injectable polyurethane-based hydrogel with multibiological functions for rapid hemostasis. Biomacromolecules. 2025;26(8):5423-5437.
-
48. Yang W, Kang X, Gao X, Zhuang Y, Fan C, Shen H, et al. Biomimetic natural biopolymer-based wet-tissue adhesive for tough adhesion, seamless sealed, emergency/nonpressing hemostasis, and promoted wound healing. Adv Funct Mater. 2023;33(6):2211340.[DOI]
-
49. Zou CY, Lei XX, Hu JJ, Jiang YL, Li QJ, Song YT, et al. Multi-crosslinking hydrogels with robust bio-adhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact Mater. 2022;16:388-402.
-
50. Chen Q, Yan M, Hu A, Liang B, Lu H, Zhou L, et al. Injectable nanorobot-hydrogel superstructure for hemostasis and anticancer therapy of spinal metastasis. Nanomicro Lett. 2024;16(1):259.
-
51. Ren H, Zhang Z, Cheng X, Zou Z, Chen X, He C. Injectable, self-healing hydrogel adhesives with firm tissue adhesion and on-demand biodegradation for sutureless wound closure. Sci Adv. 2023;9(33):eadh4327.
-
52. Zheng Y, Shariati K, Ghovvati M, Vo S, Origer N, Imahori T, et al. Hemostatic patch with ultra-strengthened mechanical properties for efficient adhesion to wet surfaces. Biomaterials. 2023;301:122240.
-
53. Weng H, Jia W, Li M, Chen Z. New injectable chitosan-hyaluronic acid based hydrogels for hemostasis and wound healing. Carbohydr Polym. 2022;294:119767.
-
54. Min WK, Kim JE, Choi DJ, Park EJ, Heo J. Clinical and radiological outcomes between biportal endoscopic decompression and microscopic decompression in lumbar spinal stenosis. J Orthop Sci. 2020;25(3):371-378.
-
55. Danker W, Kelkar SS, Marston XL, Aggarwal J, Johnston SS. Real-world economic and clinical outcomes associated with current hemostatic matrix use in spinal surgery. J Comp Eff Res. 2022;11(17):1231-1240.
-
56. Krogman H, Stevens B, Kanhai M, Meroney M, Kumar S, Jafari A. Postoperative transient neurogenic claudication after lumbar endoscopic decompression: The role of floseal in minimally invasive spine surgery. Cureus. 2024;16(11):e73083.
-
57. Kim JE, Yoo HS, Choi DJ, Park EJ, Hwang JH, Suh JD, et al. Effectiveness of gelatin-thrombin matrix sealants (Floseal®) on postoperative spinal epidural hematoma during single-level lumbar decompression using biportal endoscopic spine surgery: Clinical and magnetic resonance image study. Biomed Res Int. 2020;2020:4801641.
-
58. Ashraf H, Low N, Spiro C, Keong B. Floseal-induced small bowel obstruction. J Surg Case Rep. 2022;2022(9):rjac444.
-
59. Bozkurt I, Kazanci A, Gurcan O, Gurcay AG, Arikok AT, Bavbek M. Spinal epidural fibrosis following hemostatic agent employment. Br J Neurosurg. 2023;37(2):137-141.
-
60. Abe T, Miyazaki M, Sako N, Kanezaki S, Hirakawa M, Kawano M, et al. Efficacy of gelatin-thrombin matrix sealants for blood loss in single-level transforaminal lumbar interbody fusion. Medicine. 2023;102(36):e34667.
-
61. Park JH, Park S, Choi SA. Incidence and risk factors of spinal epidural hemorrhage after spine surgery: A cross-sectional retrospective analysis of a national database. BMC Musculoskelet Disord. 2020;21(1):324.
-
62. Soejima Y, Arizono T, Bekki H, Inokuchi A, Izumi T, Imamura R, et al. Factors affecting postoperative spinal epidural hematoma and the optimal order of vertebral body decompression in multivertebral microendoscopic laminectomy. Cureus. 2022;14(5):e25404.
-
63. Yan H, Gao M, Zhang Y, Wang H, Zhu Y, Zhou T, et al. Use of fluid gelatin in lumbar spinal stenosis undergoing unilateral biportal endoscopic: A prospective, randomized controlled trial. Orthop Surg. 2025;17(5):1340-1348.
-
64. Park SM, Kang DR, Lee JH, Jeong YH, Shin DA, Yi S, et al. Efficacy and safety of a thrombin-containing collagen-based hemostatic agent in spinal surgery: A randomized clinical trial. World Neurosurg. 2021;154:e215-e221.
-
65. An S, Kwon WK, Choi I, Lee JB, Kim J, Hur JW. Evaluating the efficacy and safety of hemofence (thorombin cross-linked sodium hyaluronate gel matrix) in hemostasis for intractable exudative bleeding in spinal surgery: A multicenter, randomized, phase III clinical trial. Neurospine. 2024;21(3):1004-1013.
-
66. Gomez-Florit M, Pardo A, Domingues RMA, Graça AL, Babo PS, Reis RL, et al. Natural-based hydrogels for tissue engineering applications. Molecules. 2020;25(24):5858.
-
67. Ovcharenko EA, Seifalian A, Rezvova MA, Klyshnikov KY, Glushkova TV, Akenteva TN, et al. A new nanocomposite copolymer based on functionalised graphene oxide for development of heart valves. Sci Rep. 2020;10(1):5271.
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite