Lingzhu Chen, State Key Laboratory of Green Pesticide, Guizhou University, Guiyang 550025, Guizhou, China. E-mail: lzchen@gzu.edu.cn
Abstract
This review systematically summarizes recent advances in N-heterocyclic carbene (NHC)-catalyzed asymmetric synthesis of non-central chiral compounds. The key synthetic strategies include oxidative and redox-neutral acylation reactions, LUMO-activation-mediated conjugate additions, cyclization reactions, benzoin/Stetter reactions, and imine activation processes. In this work, the versatility of NHCs in asymmetric synthesis is highlighted by their capacity to achieve high stereoselectivity across a wide range of substrates. These advances provide valuable insights for applications in chiral drug development, materials science, and catalysis.
Graphical Abstract
Keywords
1. Introduction
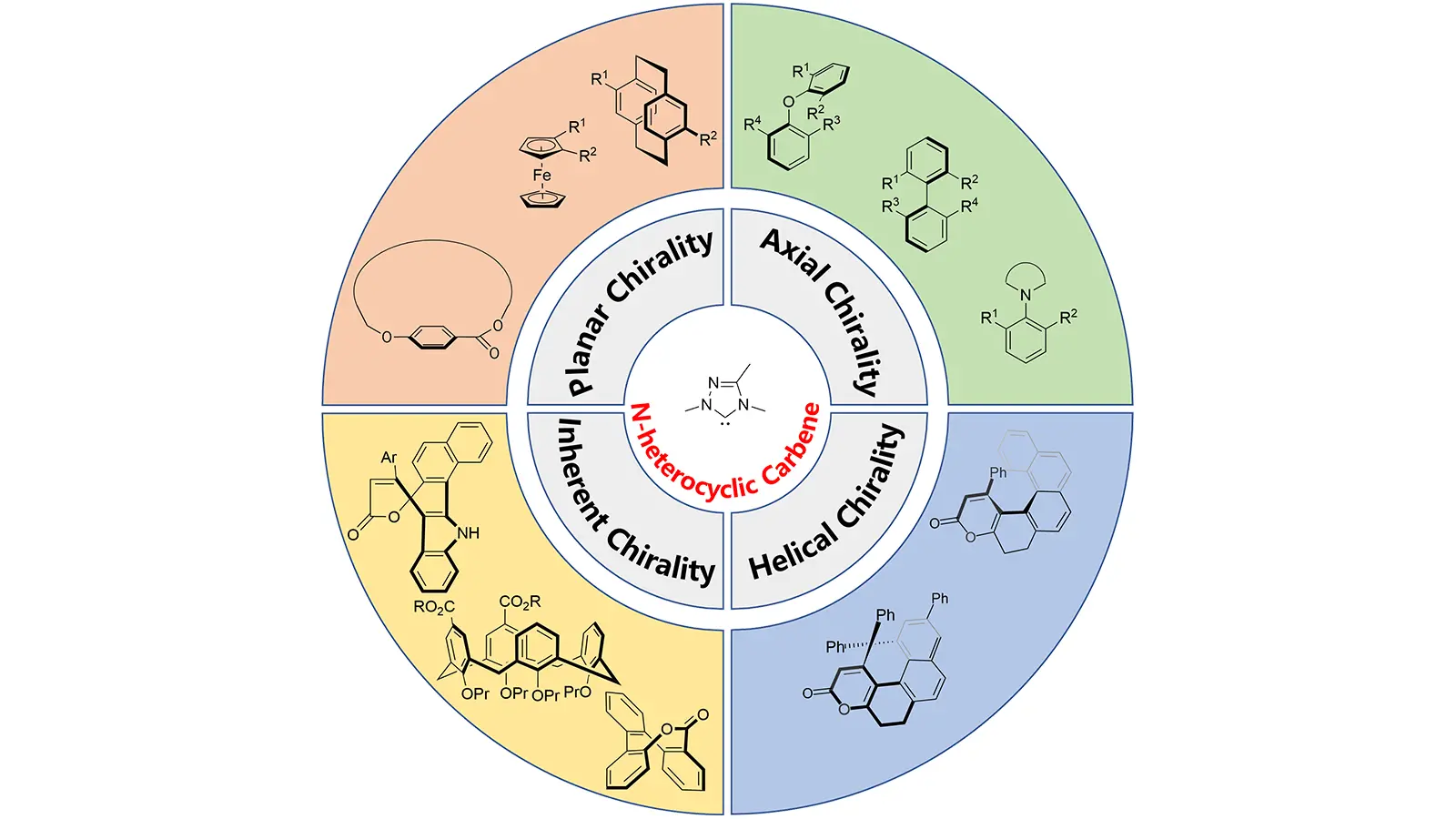
Non-central chiral compounds, including axially chiral[1], planar chiral[2], inherently chiral[3], and helically chiral molecules[4], have attracted considerable attention due to their significant roles in modern chemical science. These compounds exhibit unique biological activities and physicochemical properties, making them indispensable scaffolds in pharmaceuticals[5], agrochemicals[6,7], and functional materials[8]. Besides, their unusual stereochemical architectures create distinct three-dimensional environments that can be exploited in asymmetric catalysis. As catalysts or ligands, non-central chiral frameworks provide stereochemical spaces and recognition patterns that are difficult to achieve with conventional central chiral motifs, thereby enabling new opportunities for catalyst design and stereocontrol[9].
Despite their importance, the efficient synthesis of non-central chiral compounds remains a considerable challenge[10]. Conventional asymmetric approaches, such as transition-metal catalysis, chiral resolution, and the use of stoichiometric chiral auxiliaries, are often constrained by narrow substrate scope, reliance on precious metals, harsh reaction conditions, and only moderate enantioselectivity. These constraints underscore the urgent need for complementary and sustainable methodologies capable of constructing diverse non-central chiral architectures with high efficiency and stereocontrol.
Over the past two decades, N-heterocyclic carbenes (NHCs) have emerged as a powerful class of organocatalysts in asymmetric synthesis[11]. Their catalytic versatility arises from their ability to activate diverse substrates through the generation of reactive intermediates, including Breslow intermediates, acylazoliums, and homoenolates. These intermediates facilitate a broad array of transformations, such as acylation, benzoin and Stetter reactions, conjugate additions, annulations, cyclizations, and imine activations. Importantly, the steric and electronic tunability of NHCs enables precise stereocontrol, making them particularly effective for the asymmetric construction of non-central chiral molecules. Strategic approaches such as desymmetrization, kinetic resolution (KR), and dynamic kinetic resolution (DKR) further expand the utility of NHC catalysis, delivering high enantioselectivity across various substrates.
This review offers a comprehensive overview of recent progress in NHC-catalyzed asymmetric synthesis of non-central chiral compounds, with an emphasis on literature reported from 2014 to 2025. We discuss representative methodologies for constructing four major categories of non-central chirality: axial, planar, inherent, and helical. Each category is analyzed in terms of reaction design, mechanistic understanding, and synthetic applications.
By consolidating recent progress and identifying emerging trends, this review aims to provide a valuable reference for researchers in the field and to stimulate further innovation in the synthesis of non-central chiral molecules.
2. NHC-Catalyzed Asymmetric Synthesis of Axially Chiral Compounds
2.1 Construction of axially chiral compounds via acylation reactions
2.1.1 Construction of axially chiral compounds via oxidative acylation reactions
Axially chiral biaryl compounds commonly function as ligands or catalysts in enantioselective transformations. Among them, 2-amino-2'-hydroxy-1,1'-binaphthyl has emerged as a privileged motif due to its excellent stereocontrol performance and intrinsic bioactivity. In 2019, Yang et al. reported a novel NHC-catalyzed atropoenantioselective acylation of biphenols 1 (Scheme 1)[12]. This protocol employs a cascade strategy that integrates desymmetrization and KR, enabling the efficient construction of axially chiral biaryl amino alcohols 2. Aliphatic aldehydes bearing bulky substituents proved to be effective acyl donors, furnishing products with both high yield and enantioselectivity. A broad range of biaryl-type biphenols 1, including their derivatives involving electron-donating and electron-withdrawing groups, cyclohexane-fused biphenyls, indole-based biaryls, and phenyl-naphthyl biaryls, underwent smooth reactions to afford the desired products with high enantiomeric excess. Furthermore, the enantiomers of the products 2 could be readily obtained by employing the corresponding enantiomeric catalysts.
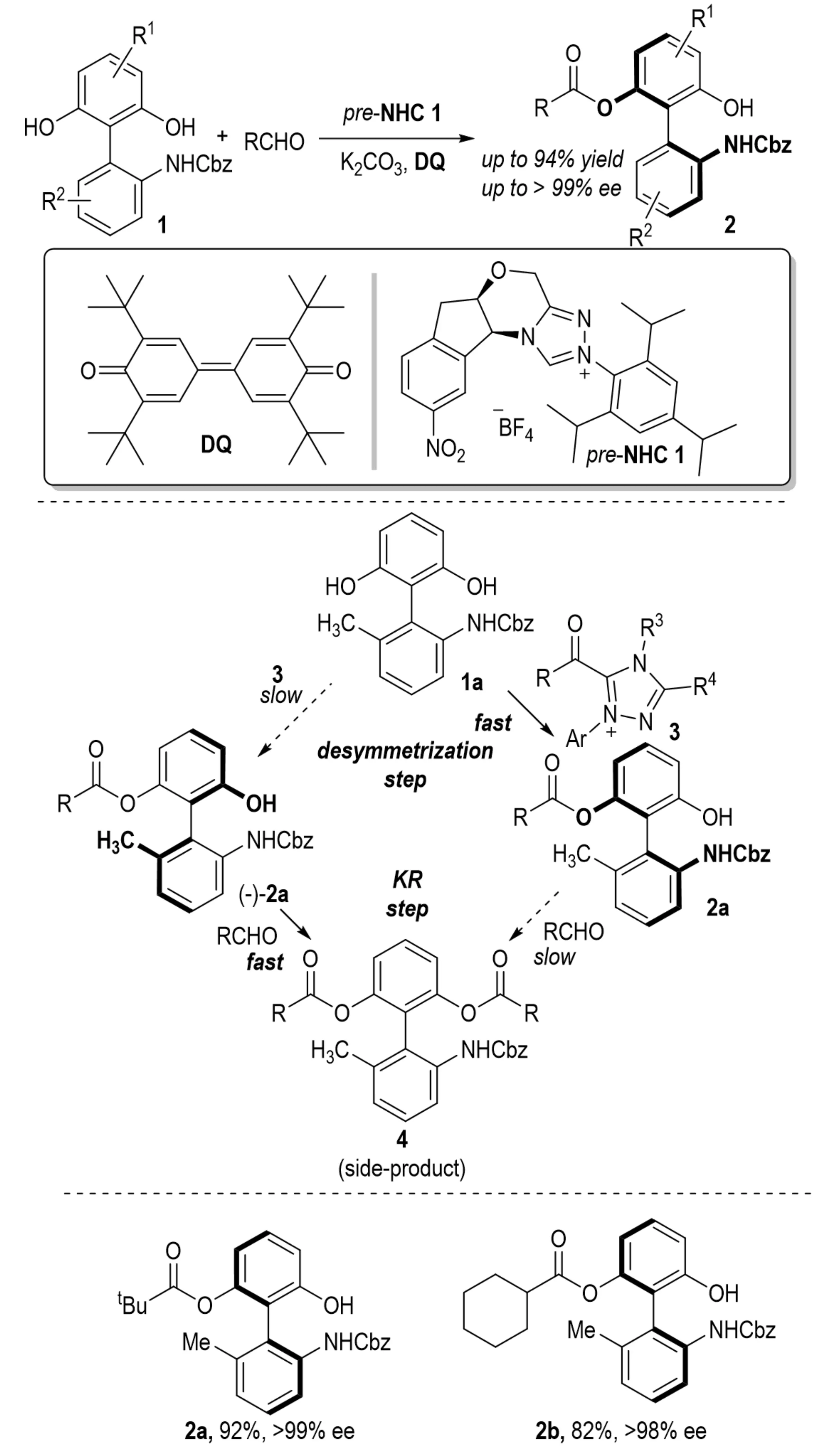
Scheme 1. Novel NHC-catalyzed atropoenantioselective acylation reaction of biphenols. NHC: N-heterocyclic carbene.
Three years later, Wu et al. extended the desymmetrization/KR cascade strategy to the asymmetric esterification of biaryl dialdehydes 5 (Scheme 2)[13]. This method afforded a series of axially chiral aldehydes 6 in good to excellent yields with high enantioselectivity.
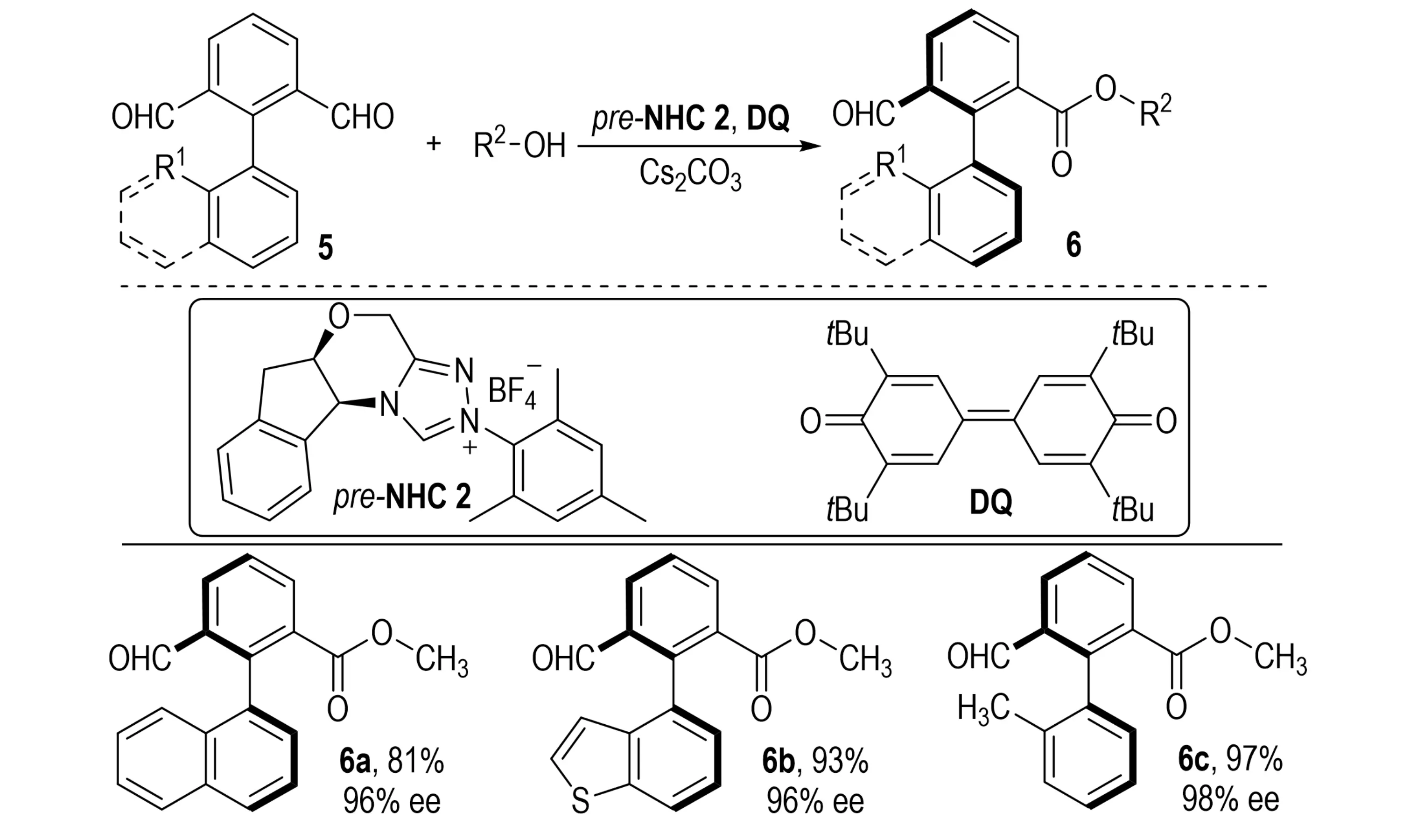
Scheme 2. Synthesis of highly enantioselective axially chiral aldehydes via NHC-catalyzed asymmetric esterification. NHC: N-heterocyclic carbene.
In 2023, Mondal et al. achieved the direct acylation of ketones through an NHC- and base-mediated DKR strategy (Scheme 3)[14]. This protocol furnished enol esters 9 in consistently high yields with excellent enantioselectivities. The transformation involves ketone–enol tautomerization, rapid interconversion of enantiomeric enol intermediates, and enantioselective acylation of the enol by an acyl azolium species 10. Subsequent one-pot oxidation generated valuable non-C2-symmetric binaphthyl derivatives. The observed high enantioselectivity was attributed to the stereodetermining interaction between the chiral acyl azolium intermediate and the transiently enol nucleophile.
Scheme 3. NHC-catalyzed enantioselective synthesis of axially chiral enol esters via dynamic kinetic resolution. NHC: N-heterocyclic carbene.
In the same year, Biju et al. realized the construction of N–N and C–O axial chirality, which are challenging due to the relatively low rotational barriers of these bonds. The N–N axial chirality was established through an NHC-catalyzed atroposelective amidation protocol (Scheme 4)[15]. In this system, aldehydes served as acyl precursors to asymmetrically functionalize quinazolinone derivative 15, providing enantioenriched 3-acylamino quinazolinones 18 featuring N–N axial chirality. This method represents the first application of NHC catalysis to the synthesis of N–N axially chiral compounds. It obviates the need for coupling or acyl transfer agents while operating under mild reaction conditions with excellent functional group tolerance. This breakthrough not only provides an efficient synthetic route to N–N axially chiral molecules, but also opens new avenues for pharmaceutical development and the preparation of chiral ligands, thereby advancing the field of axial chiral synthesis.
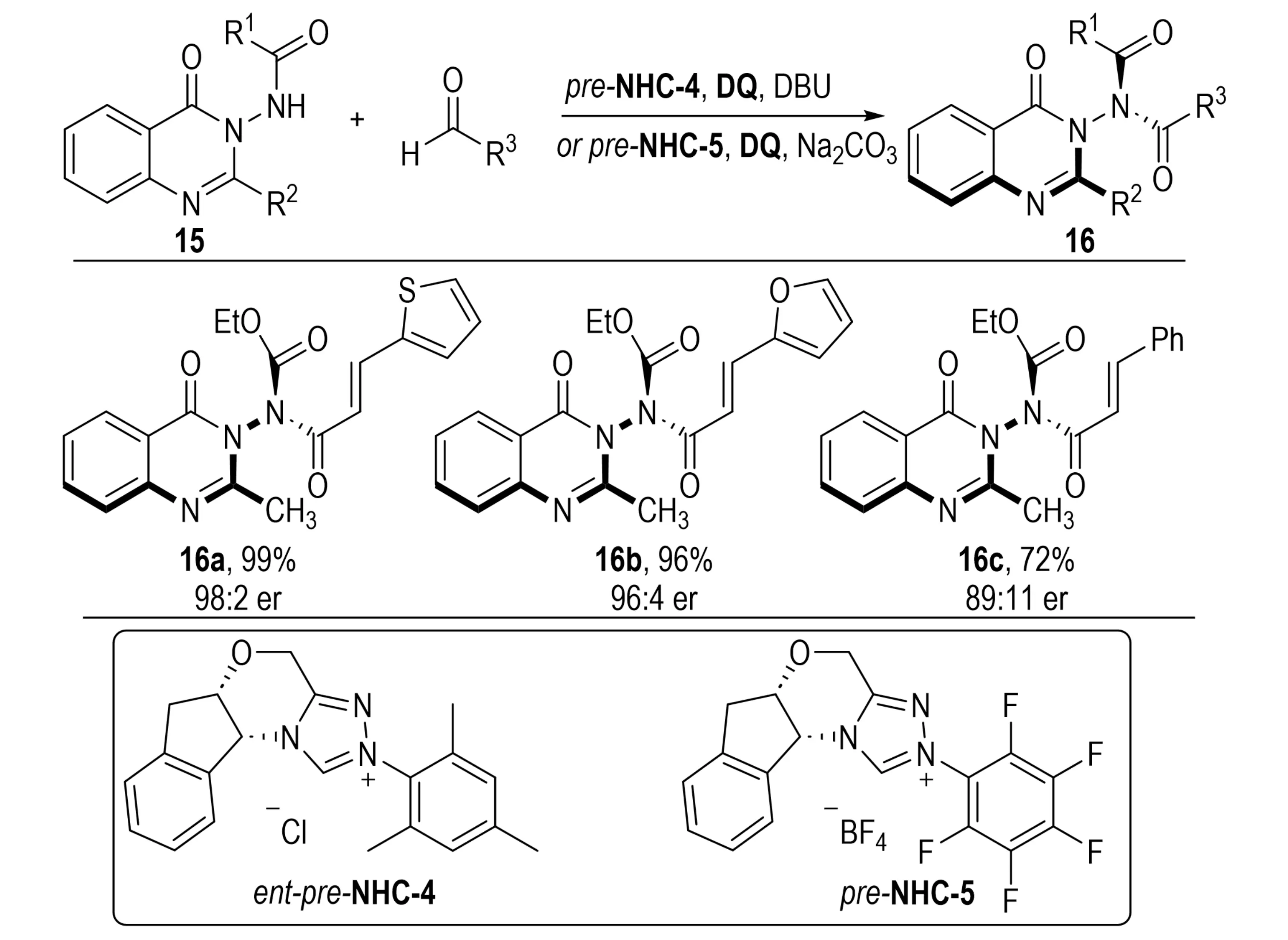
Scheme 4. NHC-catalyzed atroposelective amidation for constructing N–N axially chiral quinazolinones. NHC: N-heterocyclic carbene.
C–O axial chirality was achieved through asymmetric esterification of the meso-dialdehyde 17, which possesses two prochiral C–O axes with β-naphthol 18, affording axially chiral diaryl ether 20 in 86% yield and 98% ee (Scheme 5)[16]. In the same year, Zhou et al. reported an alternative NHC-catalyzed synthesis of axially chiral diaryl ethers using benzothiazole amines as acylation reagents[17]. Subsequently, in 2024, Li et al. realized a related transformation via a synergistic mechanism that combines desymmetrization with KR[18]. Concurrently, Wu et al.[19] introduced a “chiral induction amplification” strategy to overcome stereocontrol challenges associated with conformationally flexible dual axes. Furthermore, Liu et al.[20] demonstrated efficient chiral induction through NHC-catalyzed selective activation of dialdehyde substrates.
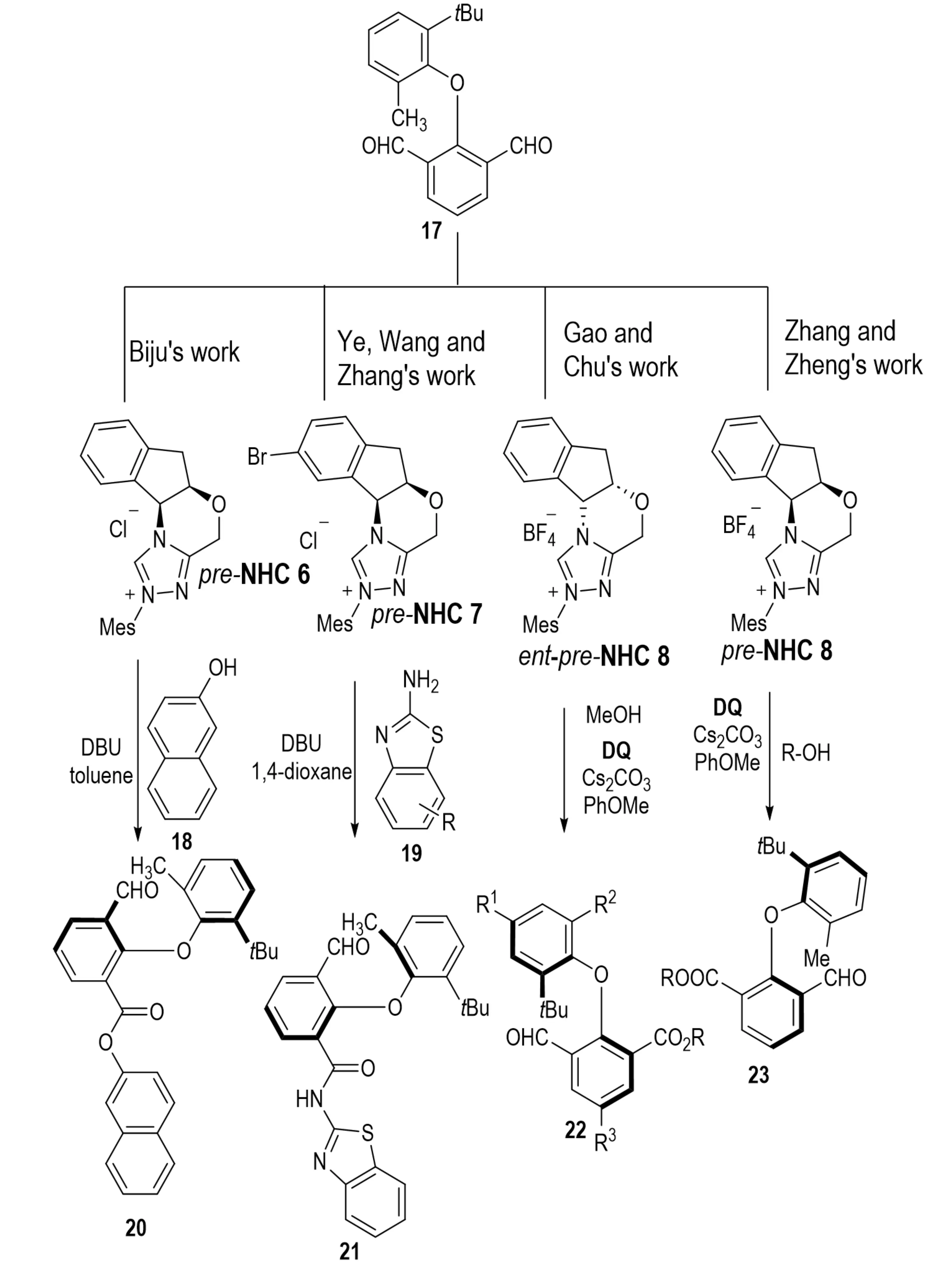
Scheme 5. Evolution of NHC-catalyzed strategies for constructing C–O axially chiral diaryl ethers. NHC: N-heterocyclic carbene.
2.1.2 Construction of axially chiral compounds via redox-neutral acylation reactions
In 2014, Lu et al. reported the first enantioselective desymmetrization/KR of racemic biaryl diols and amino alcohols 24 through an NHC-catalyzed acylation strategy (Scheme 6)[21]. In this system, aldehydes acted as acyl sources and were activated by the NHC catalyst to form a chiral acyl azolium intermediate, which selectively acylated one enantiomer of 25a, providing axially chiral biaryl alcohols 30. Both diols and amino alcohols were resolved with excellent enantioselectivity, typically affording ≥ 99% ee. This direct, economical, and efficient approach enables the asymmetric synthesis of axially chiral alcohols, characterized by simple operation, mild conditions, and diverse substrates.
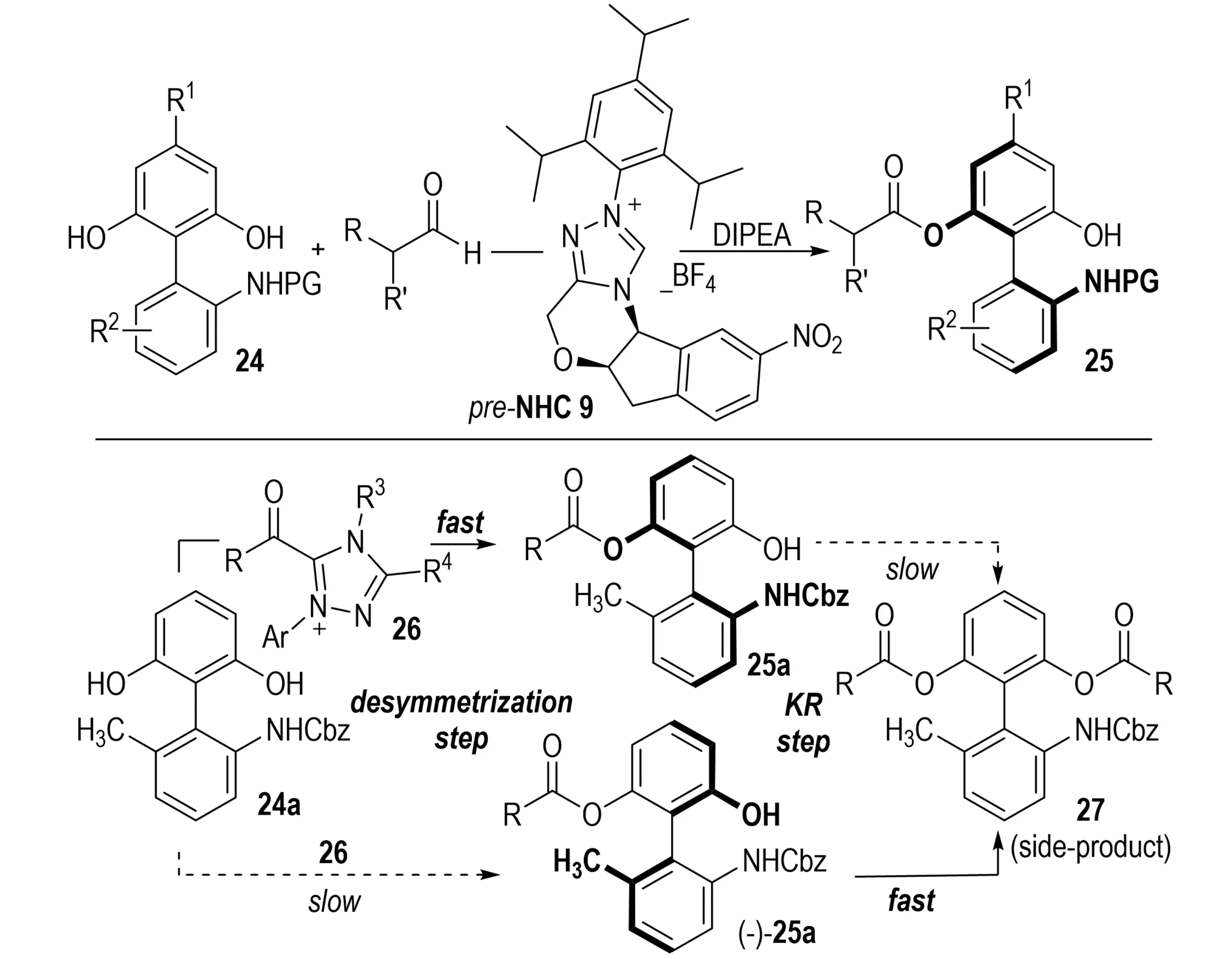
Scheme 6. NHC-catalyzed kinetic resolution of axially chiral biaryl alcohols via enantioselective acylation with an aldehyde acyl source. NHC: N-heterocyclic carbene.
In 2018, Bie et al. developed an NHC-catalyzed KR of racemic anilides rac-28 employing alkynals as acyl donors (Scheme 7)[22]. The process involved formation of a Breslow intermediate, proton transfer, and tautomerization to an enol azolium species, which was subsequently oxidized in situ to generate the key chiral acyl azolium intermediate. This electrophilic species underwent enantioselective C–O bond formation with one enantiomer of rac-28, affording acylated products 29 while recovering the slow-reacting enantiomer (S)-28 with high enantioselectivity.
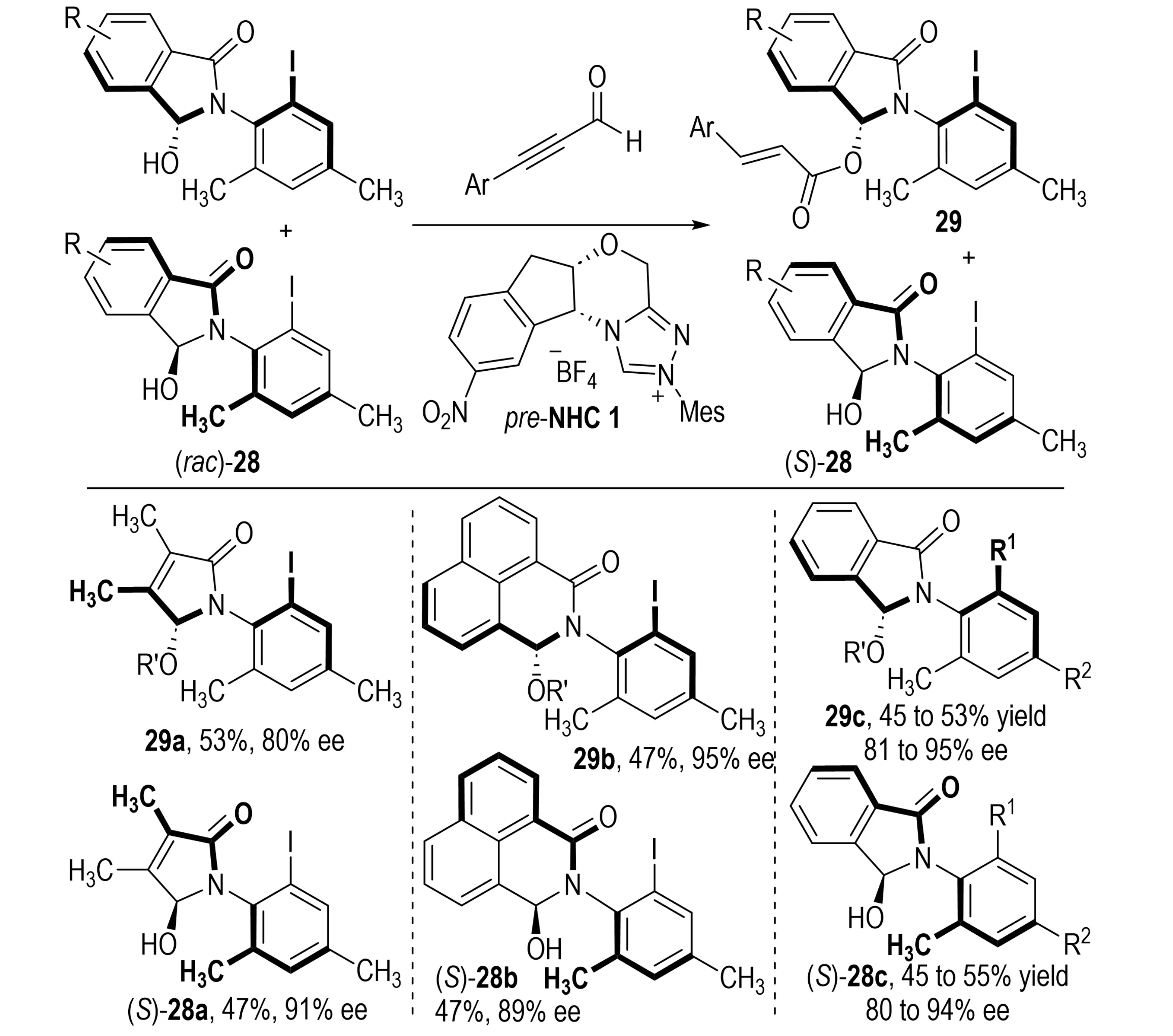
Scheme 7. NHC-catalyzed kinetic resolution of racemic anilides via enantioselective acylation. NHC: N-heterocyclic carbene.
The preparation of enantiopure NOBIN derivatives has remained a longstanding challenging[23]. In 2024, Lu et al. advanced this field by developing an efficient NHC-catalyzed asymmetric acylation of phenols (Scheme 8)[24]. Using desymmetrization/KR of achiral amino bisphenol 30, diverse NOBIN analogs 33 were obtained in yields up to 86% with excellent enantioselectivity.
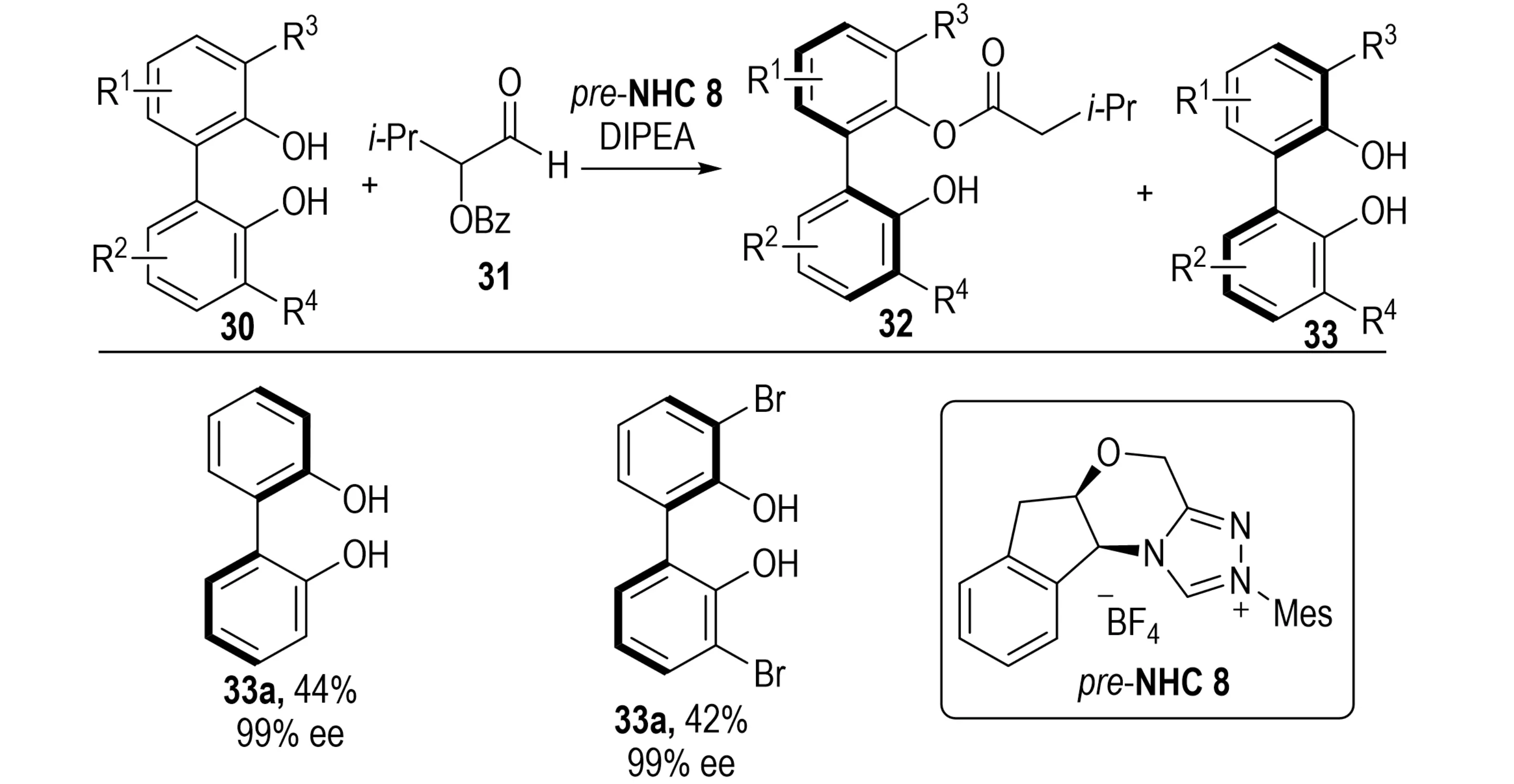
Scheme 8. NHC-catalyzed enantioselective synthesis of nobin analogs via desymmetrization/kinetic resolution of bisphenol. NHC: N-heterocyclic carbene.
In the same year, Zheng et al. reported an NHC-catalyzed chemo- and enantioselective DKR of racemic 2-arylbenzaldehydes 35 with α-bromoenals 34, providing axially chiral aldehydes 36 (Scheme 9)[25]. The transformation proceeded under mild conditions, tolerated a wide range of substituents, and delivered products in moderate to high yields with good to excellent enantioselectivities.
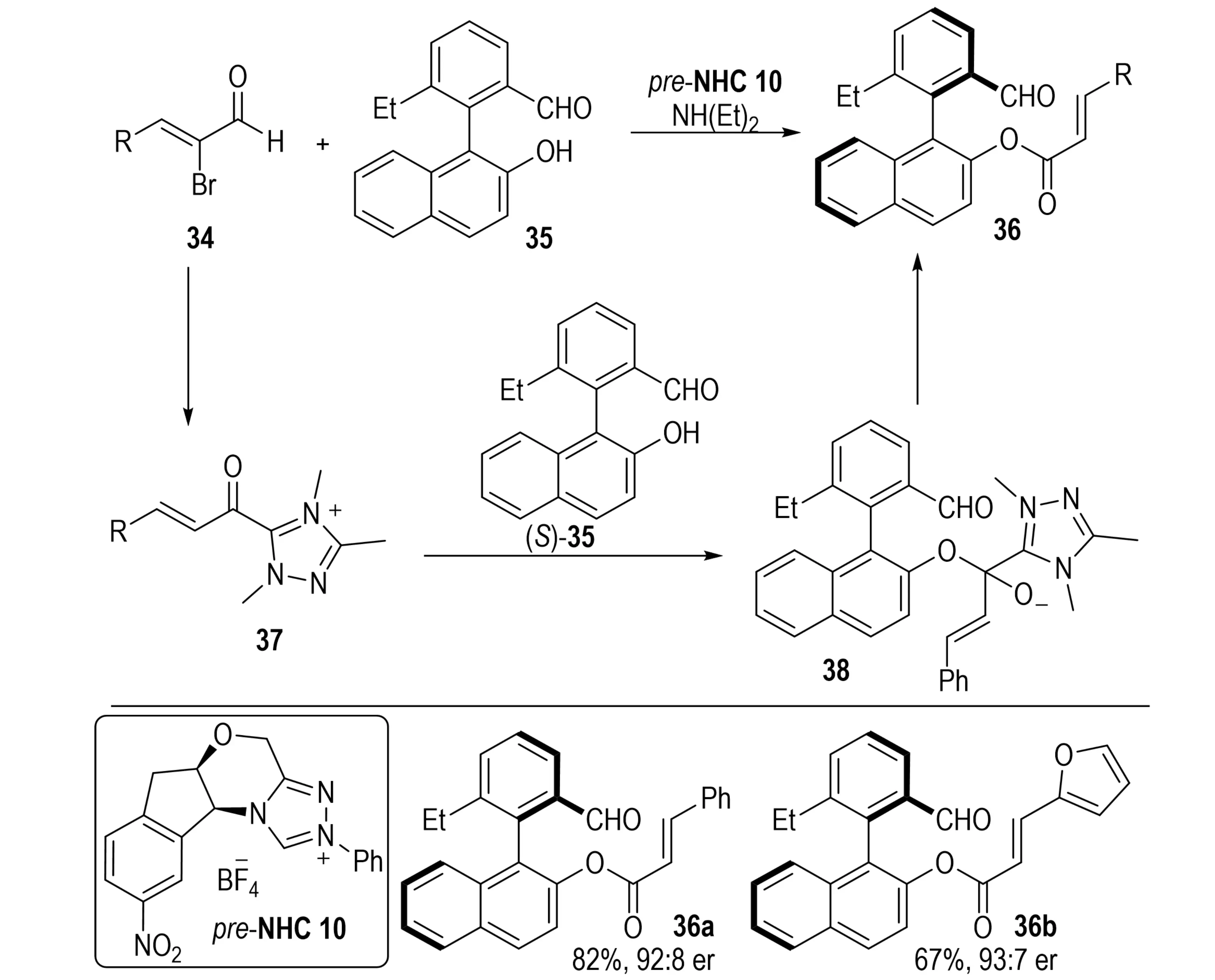
Scheme 9. NHC-catalyzed dynamic kinetic resolution for synthesis of axially chiral biaryl aldehydes from racemic 2-arylbenzaldehydes and α-bromoenals. NHC: N-heterocyclic carbene.
N-Substituted phthalimides constitute important structural motifs in pharmaceuticals and materials chemistry[26]. In 2024, Barik et al. developed an NHC-catalyzed atroposelective synthesis of N-aryl phthalimides 40 and maleimides from carboxylic acids 39 (Scheme 10)[27]. The reaction proceeds via in situ activation of phthalamic acids with pivaloyl chloride to form isoimides, which underwent NHC-mediated generation of acyl azolium intermediates, enabling asymmetric amidation. Both experimental results and density functional theory (DFT) studies confirmed the configurational stability of the resulting atropisomers through the analysis of C–N bond rotational barriers. This method achieves the atroposelective amidation of phthalamic acids, representing the first construction of C–N axial chirality via an NHC-catalyzed acid activation pathway.
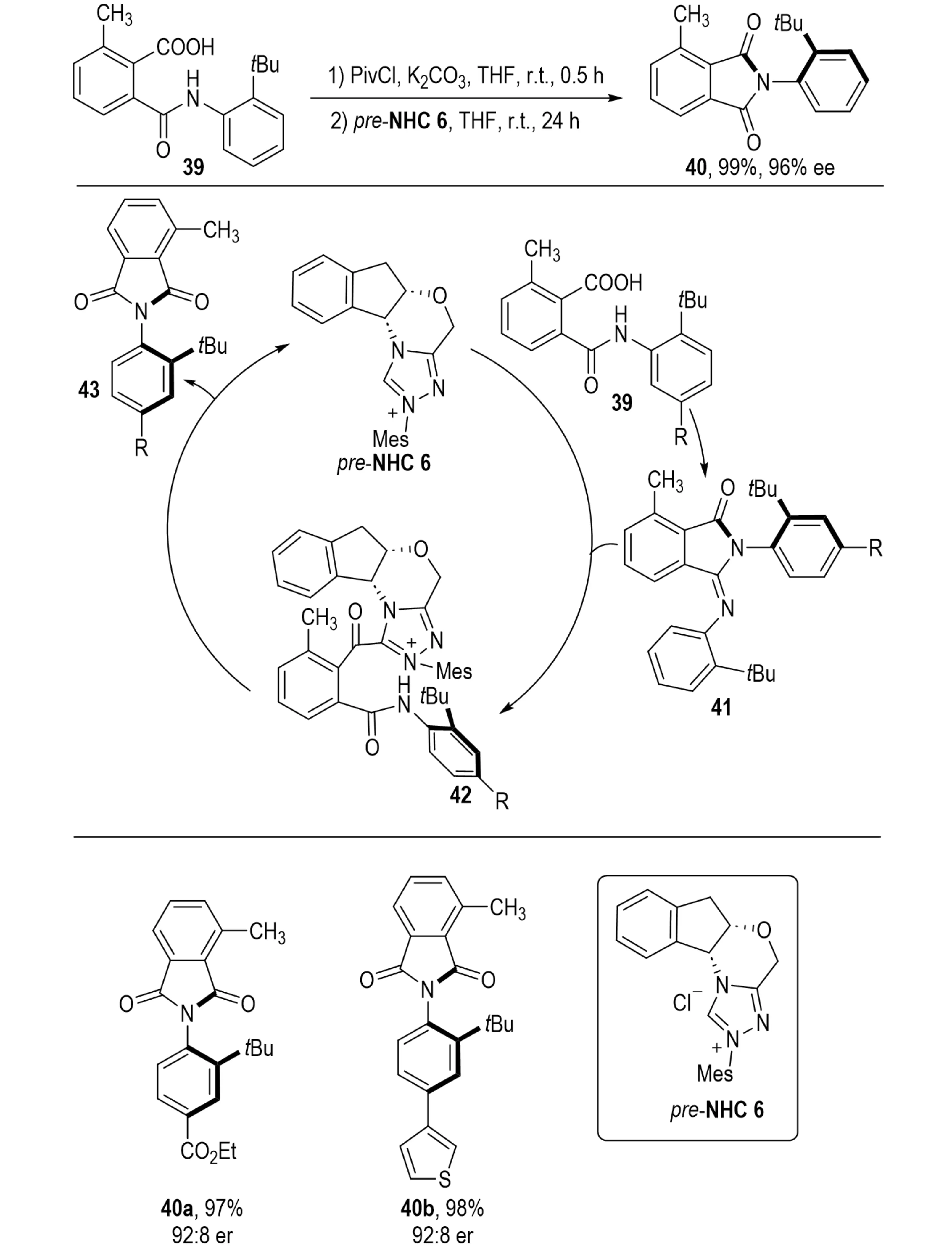
Scheme 10. NHC-catalyzed atroposelective synthesis of n-aryl phthalimides via carboxylic acid activation and asymmetric amidation. NHC: N-heterocyclic carbene.
Also in 2024, Cai et al. designed a bifunctional organocatalyst integrating a triazolium-derived NHC with an aryl amide hydrogen-bond donor (Scheme 11)[28]. This catalyst promoted the DKR of cyclic biaryl lactams 44 via atroposelective ring-opening, affording products 46 in good to excellent yields with high enantioselectivities. Mechanistic studies suggested that nucleophilic attack by the carbene generated an acyl azolium intermediate, while the amide moiety activated the substrate through hydrogen bonding and precisely positioned it near the carbene center. In a related study, Wang et al. introduced a sterically demanding monofunctional NHC catalyst to achieve stereocontrol[29]. Steric repulsion directed the carbene attack trajectory, enabling smooth formation of acyl azolium intermediates and the synthesis of axially chiral biaryls without hydrogen-bonding assistance.
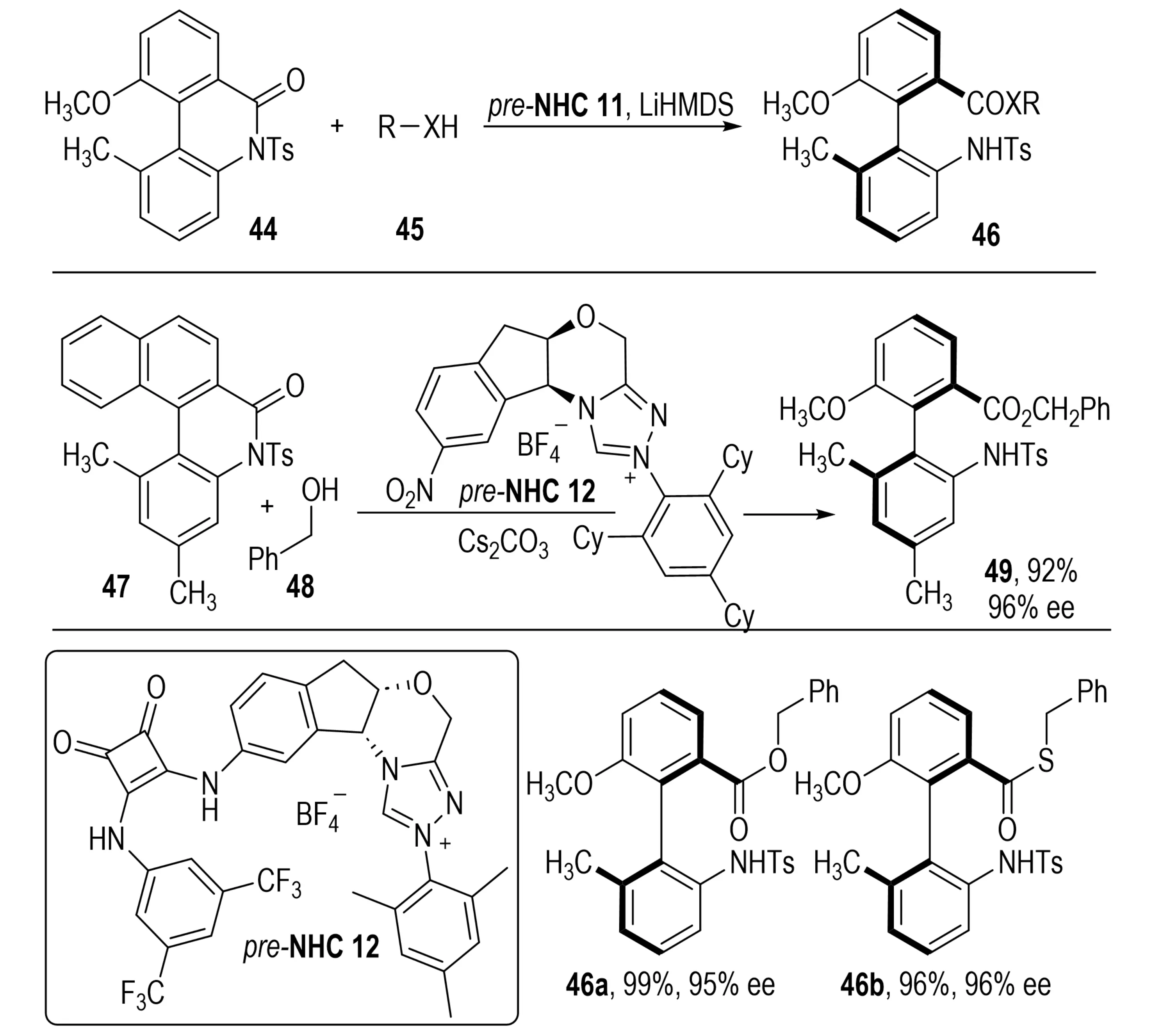
Scheme 11. NHC-catalyzed dynamic kinetic resolution of biaryl lactams via atroposelective c–n bond cleavage and ring-opening, enabling synthesis of axially chiral biaryls. NHC: N-heterocyclic carbene.
2.2 Construction of axially chiral compounds via conjugate addition enabled by LUMO activation
2.2.1 Construction of axially chiral compounds via oxidative cycloaddition enabled by LUMO activation
Urazole (1,2,4-triazoledione) is a nitrogen- and oxygen-rich heterocycle that has garnered considerable attention in the development of bioactive molecules[30]. In 2021, Jin et al. reported an NHC-catalyzed oxidative asymmetric [3+2] annulation and desymmetrization of 4-arylurazoles 50 (Scheme 12)[31]. This transformation enabled the efficient construction of a stereogenic C–N axis distal to the catalyst binding site. A wide range of axially chiral pyrazolo[1,2-a][1,2,4]triazole-3,6-dione derivatives 51 were obtained in good yields with high enantioselectivities. Thermodynamic studies further confirmed the configurational stability of these atropisomeric products by evaluating rotational barriers.
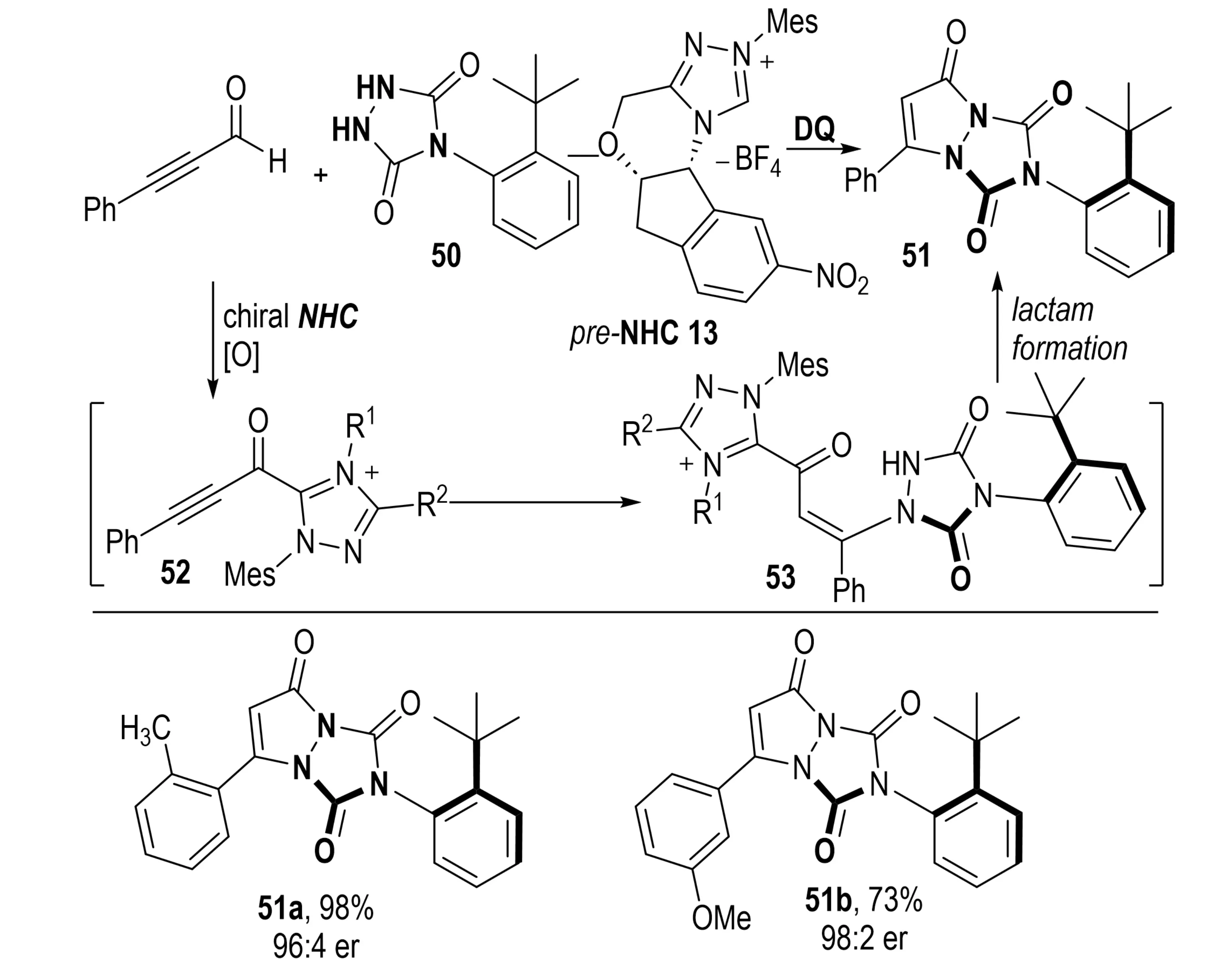
Scheme 12. NHC-catalyzed asymmetric annulation/desymmetrization of 4-arylurazoles for synthesis of axially chiral heterocycles. NHC: N-heterocyclic carbene.
In the same year, Li et al. reported an NHC-catalyzed asymmetric annulation between thioureas 54 and ynals, leading to the formation of axially chiral thiazine derivatives 55 with high enantioselectivity (Scheme 13)[32]. The key step involves nucleophilic addition of the thiourea sulfur to the β-carbon of an NHC-derived β-acetylenic acylazolium intermediate, forming a C(sp)–S bond. This is followed by a catalyst-controlled, face-selective intramolecular amidation that establishes the stereogenic C–N axis and delivers the enantioenriched thiazines.
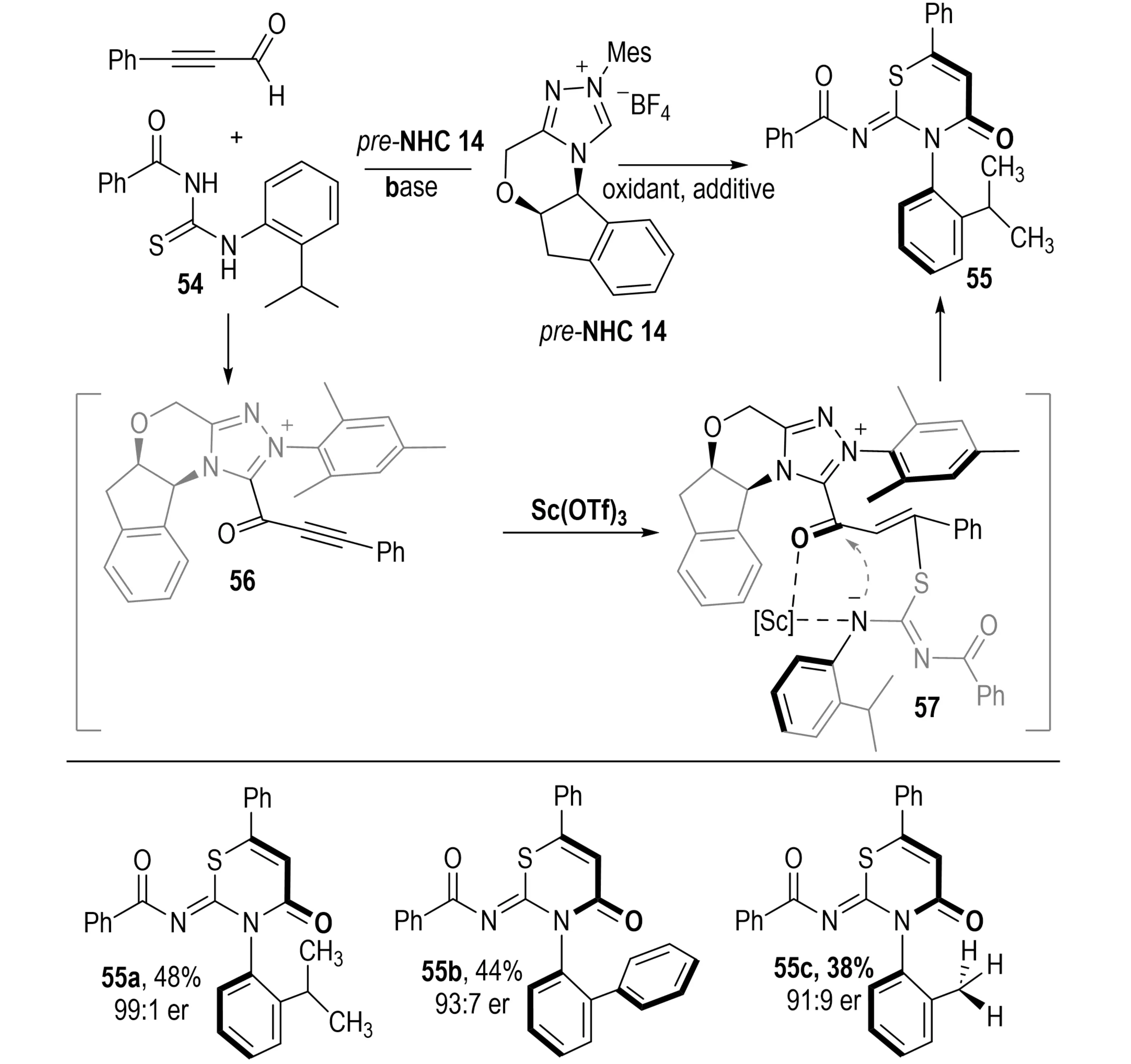
Scheme 13. NHC-catalyzed asymmetric annulation of thioureas and ynals for atroposelective synthesis of c–n axially chiral thiazines. NHC: N-heterocyclic carbene.
In 2022, Chu et al. developed an NHC-catalyzed asymmetric annulation between enals 58 and maleimide derivatives 59, providing efficient access to pyrrolo[3,4-b]pyridine derivatives 60 with configurationally stable C–N axes (Scheme 14)[33]. The process afforded products in high yields with excellent enantioselectivities. Mechanistic studies revealed that the enal reacts with the NHC to generate an α,β-unsaturated acylazolium intermediate, which undergoes 1,4-addition with a nitrogen nucleophile derived from the maleimide. Subsequent proton transfer, tautomerization, and intramolecular lactamization establish the stereogenic C–N axis, followed by in situ oxidation to furnish the aromatized product.
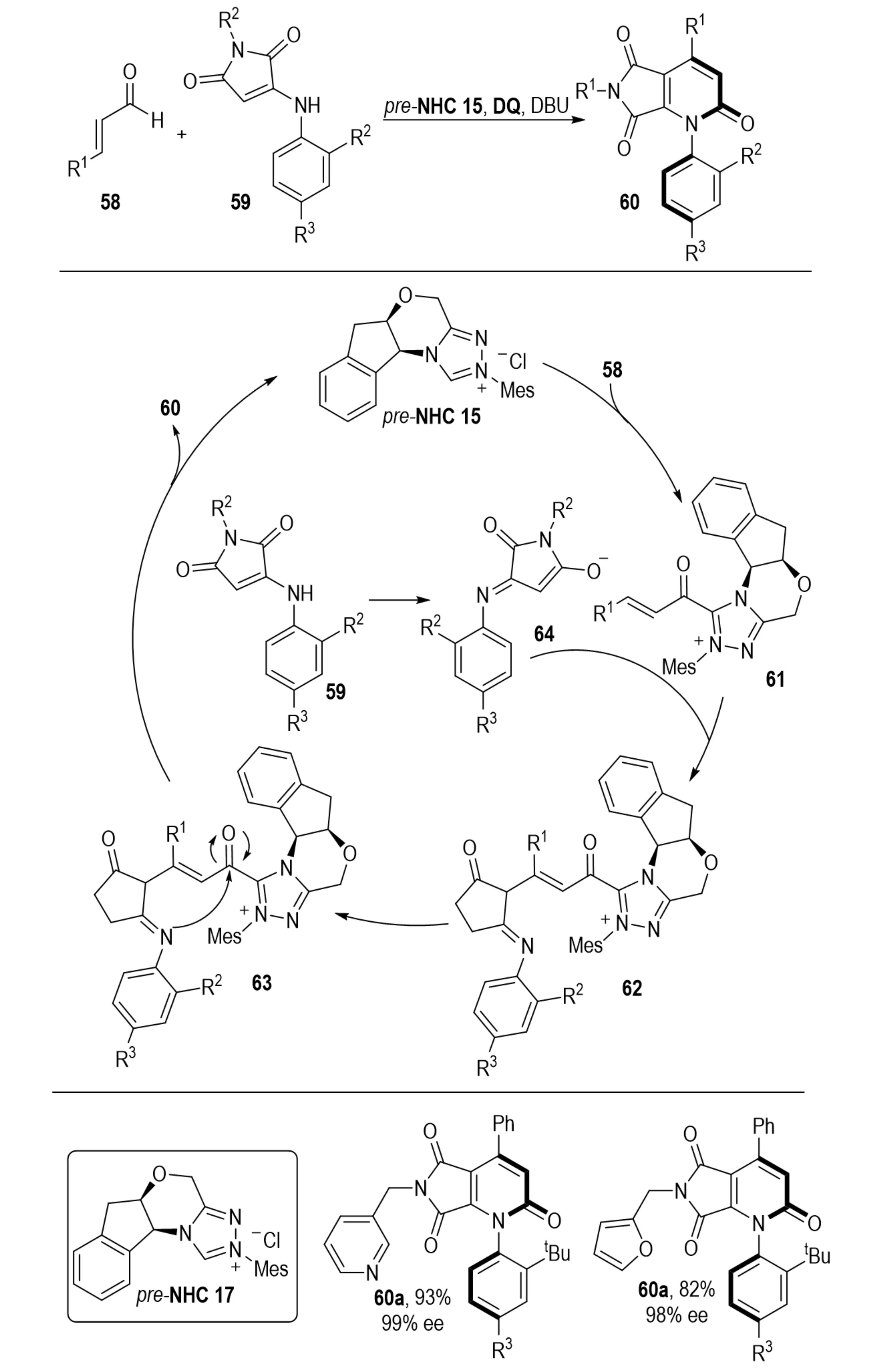
Scheme 14. NHC-catalyzed asymmetric annulation of enals and maleimides for synthesis of c–n axially chiral pyrrolo[3,4-b]pyridines. NHC: N-heterocyclic carbene.
In 2018, Xu et al. reported an NHC-catalyzed atroposelective [4+2] annulation between α-aryl ketone 66 and unsaturated aldehyde 65, affording axially chiral arenes 67 (Scheme 15)[34]. The reaction proceeds through the formation of alkynyl acyl azolium intermediates, which undergo 1,6-addition, intramolecular aldol reaction, β-lactone formation, and subsequent decarboxylation/aromatization. This method enabled polysubstituted axially chiral arenes in moderate to high yields, with excellent enantioselectivity and remarkable configurational stability. Notably, this work represents the first NHC-catalyzed intermolecular atroposelective de novo synthesis of arenes, demonstrating broad substrate scope, high stereoselectivity, and operational simplicity. Importantly, it addresses the challenging stereocontrol over axially chiral centers with minimal steric differentiation. Additionally, the reaction’s compatibility with electrochemical oxidation enhances its applicability, offering an efficient strategy for synthesizing axially chiral ligands, catalysts, and pharmaceutical molecules. Overall, this approach significantly extends the synthetic potential of NHC-mediated asymmetric organocatalysis.
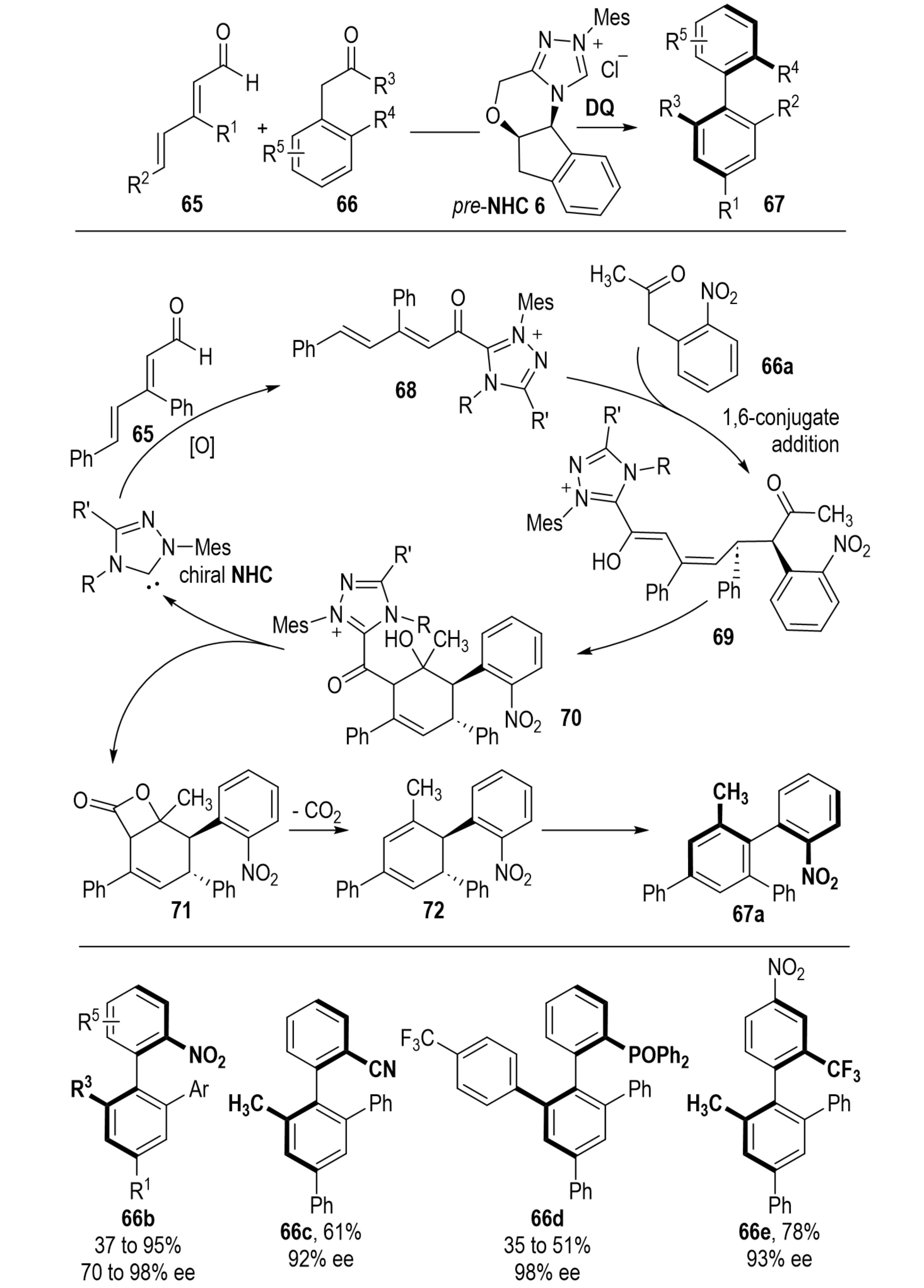
Scheme 15. NHC-catalyzed atroposelective synthesis of biaryls via formal [4+2] cycloaddition. NHC: N-heterocyclic carbene.
In 2022, Wang et al. demonstrated an NHC-catalyzed reaction between conjugated dienals and α-aryl ketones 73 to access axially chiral biaryls 74 (Scheme 16)[35]. The transformation involves in situ generation of unsaturated acyl azolium intermediates, which undergo sequential 1,6-addition, intramolecular aldol condensation, decarboxylation, and oxidative aromatization. The stereochemical outcome is directed by a central-to-axial chirality transfer process. By varying the aryl substituents on the dienal or ketone, both enantiomers of the product could be obtained under the same catalytic conditions. In 2023, Zhang et al. extended this approach to an oxidative [3+3] annulation between indole-1-pyruvate esters 75 and enals, affording axially chiral 5-indol-1-yl pyran-2-ones 76 with N–C axial chirality[36].
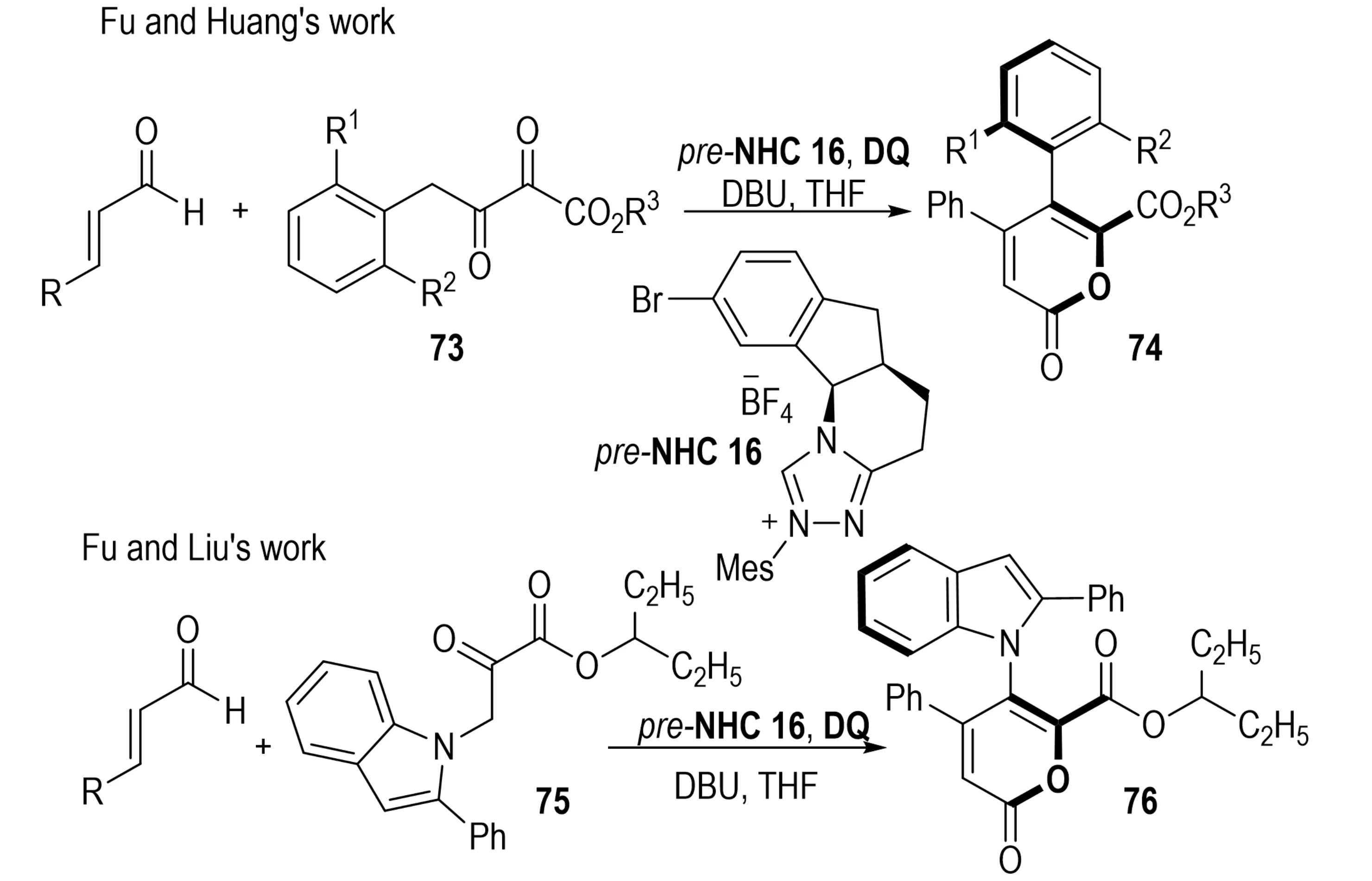
Scheme 16. NHC-catalyzed atroposelective synthesis of biaryls via central-to-axial chirality transfer. NHC: N-heterocyclic carbene.
In 2021, Zhang et al. developed an NHC-catalyzed asymmetric reaction between enals and 2-benzyl-benzothiophene/benzofuran-3-carbaldehydes 77, delivering tetra-ortho-substituted axially chiral benzothiophene- and benzofuran-fused biaryls 78 (Scheme 17)[37]. The cascade transformation proceeds through a sequence of [2+4] annulation, decarboxylation, and oxidative aromatization, facilitating the central-to-axial chirality transfer and thus providing an efficient route to sterically demanding axially chiral biaryls.
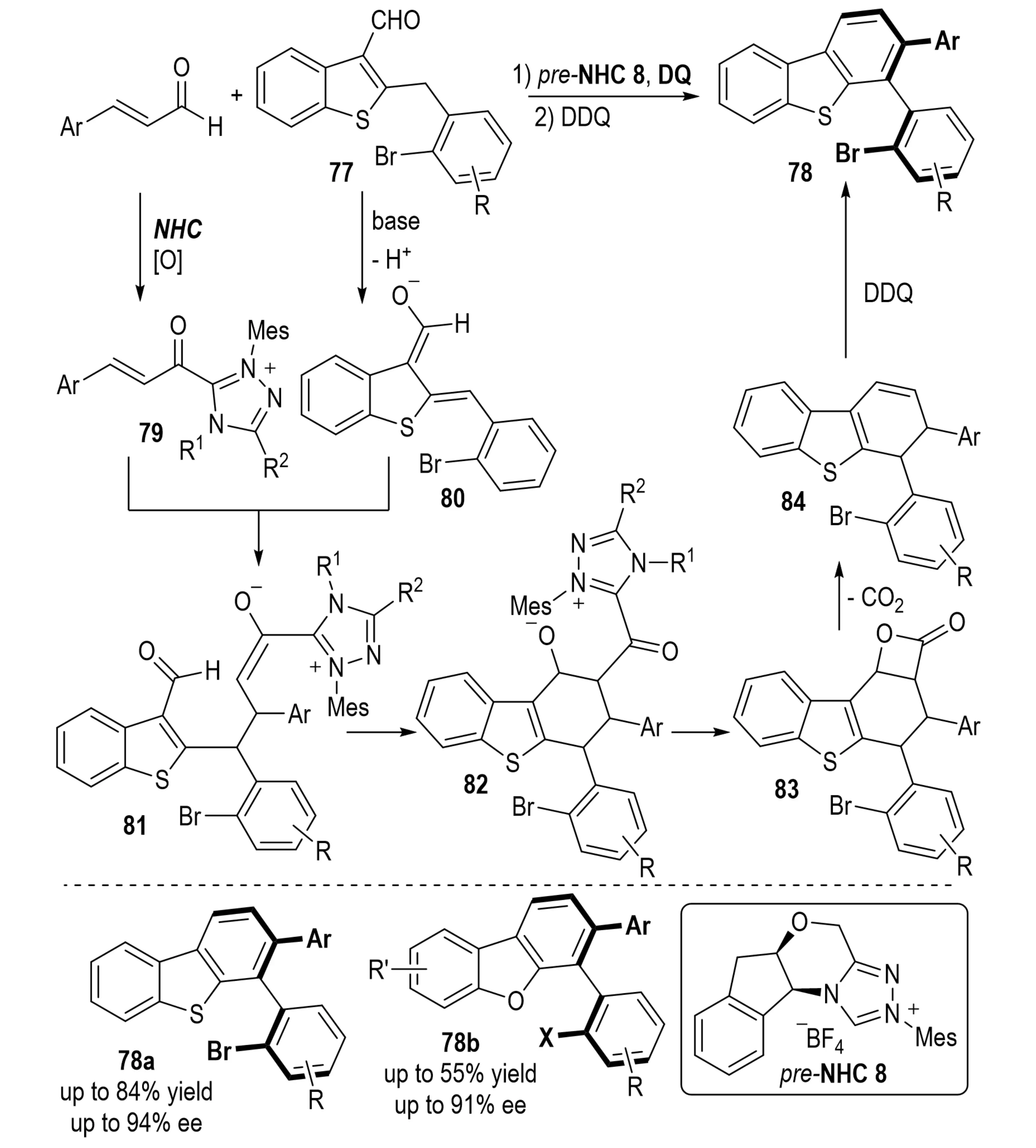
Scheme 17. NHC-catalyzed atroposelective synthesis of tetra-ortho-substituted biaryls via central-to-axial chirality transfer. NHC: N-heterocyclic carbene.
In 2022, Barik et al. reported an NHC-catalyzed kinetic resolution of racemic N-aryl aminomaleimides 85 (Scheme 18)[38]. The reaction with 2-bromoenals generated α,β-unsaturated acylazoliums, which underwent a [3+3] annulation. In this process, one enantiomer was selectively converted into fused dihydropyridinones 87 featuring both axial and central chirality, while the other one was recovered with high enantiopurity.
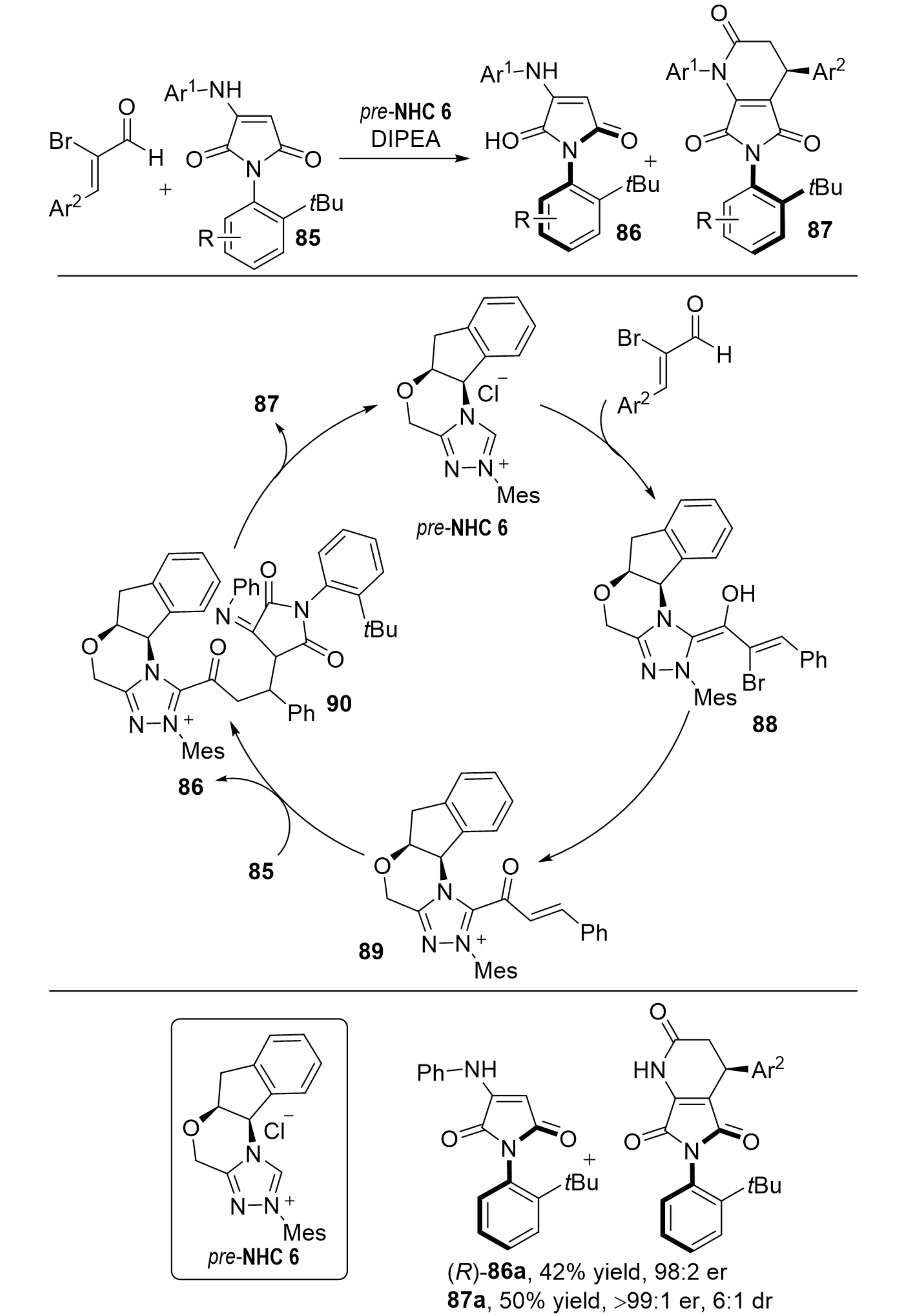
Scheme 18. NHC-catalyzed kinetic resolution via [3+3] annulation for the synthesis of C–N axially chiral compounds. NHC: N-heterocyclic carbene.
In 2022, Yan et al. reported an NHC-catalyzed three-component synthesis of axially chiral styrenes (Scheme 19)[39]. This process employed ynals 92, sulfinic acids, and phenols 91, proceeding through a 1,4-addition of sulfinate anions to NHC-activated acetylenic acylazolium intermediates, followed by E-selective protonation. The products were obtained in good to excellent yields with outstanding enantioselectivity and excellent E/Z selectivity.
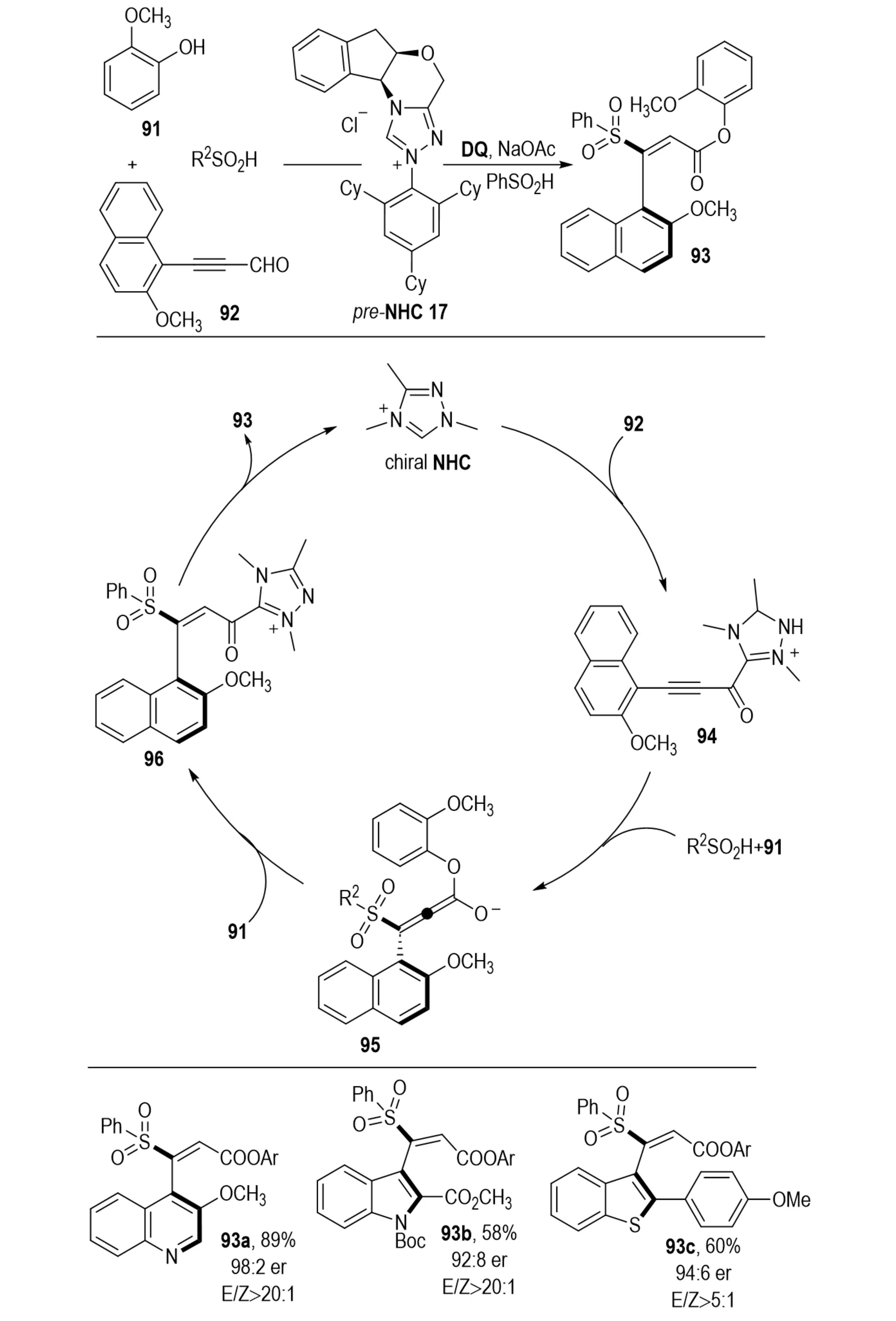
Scheme 19. Strategic enantioselective synthesis of configurationally stable axially chiral styrenes via nhc catalysis.
In 2023, Li et al. developed an NHC-catalyzed asymmetric [3+3] annulation that delivered dihydropyridinones 98 bearing both C–N axial and central chirality (Scheme 20)[40]. This transformation demonstrated high enantioselectivity, with stereocontrol directed by the chiral NHC catalyst. In 2024, Ranganathappa et al. reported an oxidative NHC-catalyzed [3+3] annulation between indole- or pyrrole-derived enamines 97 and α,β-unsaturated aldehydes, affording dihydropyridinone-fused heterocycles 100 that possess N–N axial and central chirality in a single step[41].
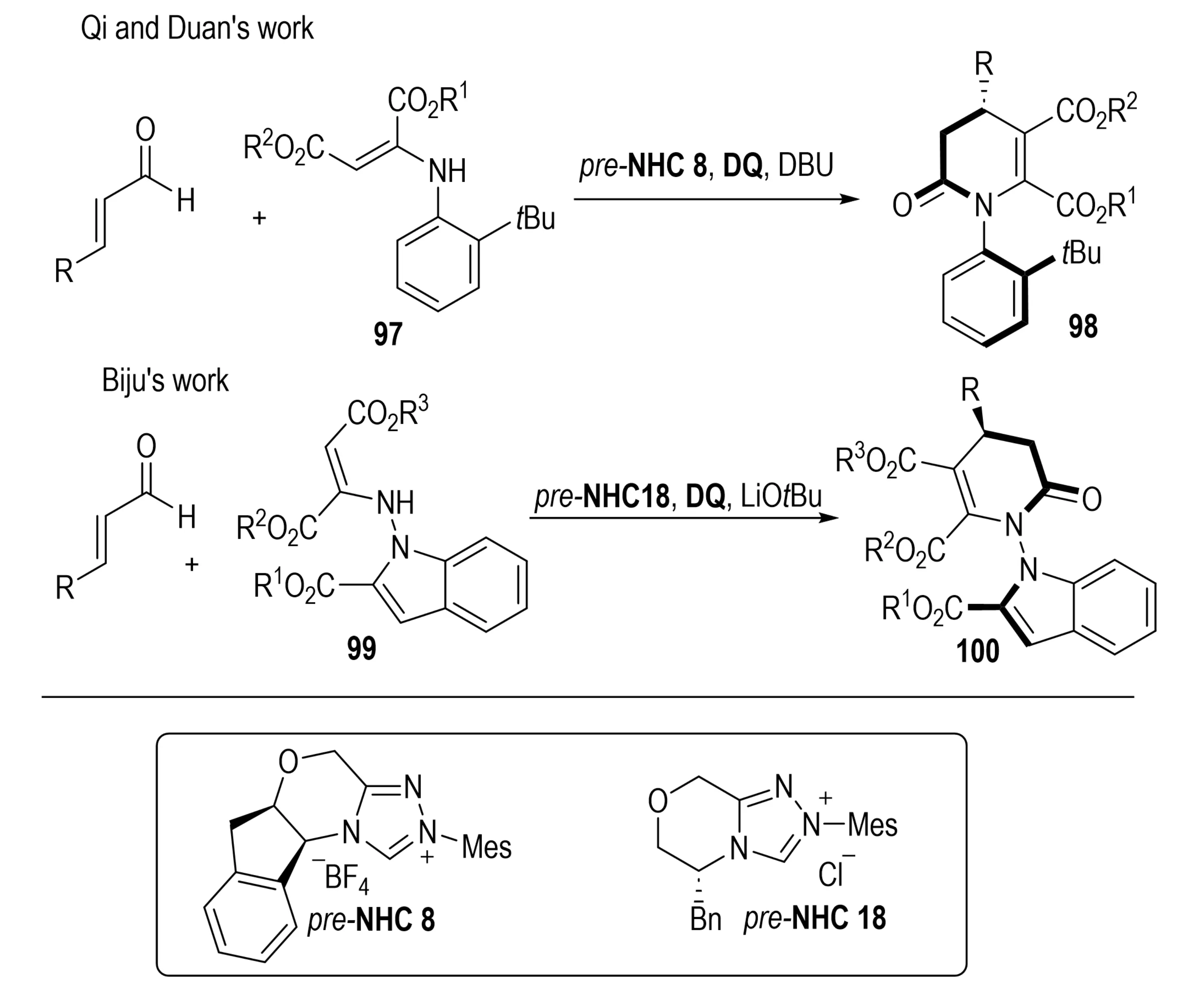
Scheme 20. NHC-catalyzed atroposelective and enantioselective [3+3] annulation for concurrent construction of C–N axial and central chirality. NHC: N-heterocyclic carbene.
In 2022, Zhang et al. reported an NHC-catalyzed redox-neutral [3+3] cyclization that directly constructed triaryl α-pyrones 102 bearing two contiguous chiral axes (Scheme 21)[42]. In the same year, Zhang et al. developed an oxidative NHC-catalyzed [3+3] annulation between 2-aryl ketones 101 and ynals 103, giving triaryl-2-pyrones with either monoaxial or contiguous diaxial chirality[43]. The stereochemical outcome was controlled by the chiral environment of the NHC catalyst; however, sterically hindered ynals were prone to racemization. In 2023, Wang et al. further demonstrated the synthesis of enantioenriched pyrano[3,2-b]indol-2-ones 109 through an NHC-catalyzed [3+3] annulation of indol-3-ones 108 with ynals 107[44].
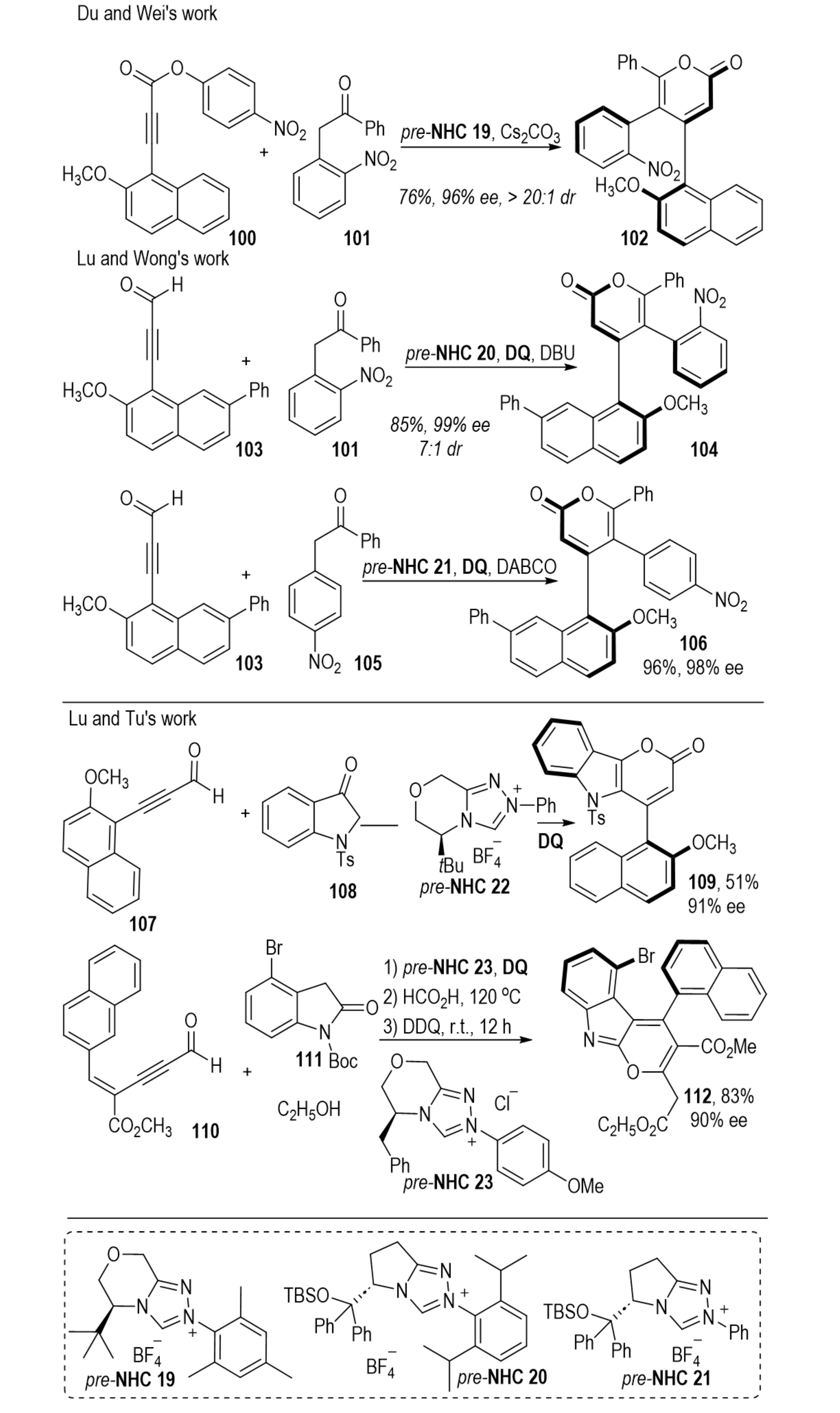
Scheme 21. NHC-catalyzed enantioselective [3+3] annulation for the synthesis of axially chiral triaryl-2-pyrones and pyranoindolones. NHC: N-heterocyclic carbene.
In 2024, Wang et al. reported an oxidative NHC-catalyzed [3+3] annulation that efficiently constructed N–N axially chiral pyrrole 114 and indole derivatives 116, simultaneously establishing contiguous N–N axial chirality and central chirality (Scheme 22)[45]. High enantioselectivity was achieved through aminoindanol-derived triazolium salts, whose bulky N-mesityl and trityl-like substituents imposed steric restrictions on key intermediates and directed nucleophilic attack from a defined enantioface.
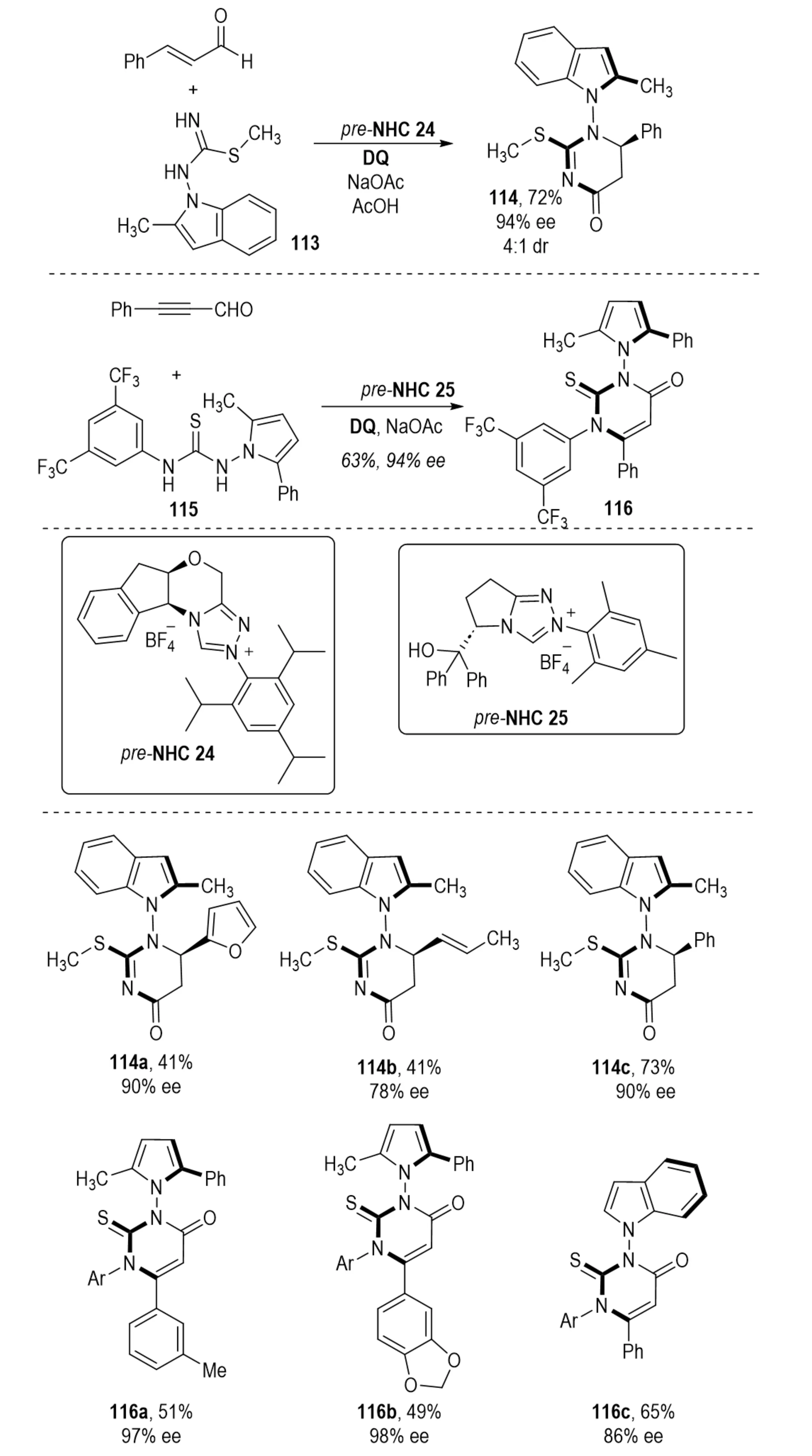
Scheme 22. NHC-catalyzed enantioselective synthesis of N-N axially chiral heterocycles with concomitant central chirality via [3+3] annulation. NHC: N-heterocyclic carbene.
2.2.2 Construction of axially chiral compounds via redox-neutral cycloaddition enabled by LUMO activation
The atroposelective construction of heterobiaryls remains particularly challenging[46]. For instance, α-Carbolines represent a privileged class of scaffolds with diverse pharmacological activities, yet effective methodologies for their asymmetric synthesis are scarce[47]. In 2021, Ma et al. developed an NHC-catalyzed asymmetric formal [3+3] annulation between 4-nitrophenyl 3-arylpropiolates 117 and 2-sulfonamidoindolines 118, affording axially chiral 4-aryl α-carbolines 120 (Scheme 23)[48]. DFT studies indicated that the observed enantioselectivity stems primarily from synergistic lone pair–π interactions and C–H···N hydrogen bonding within the stereodetermining transition state.
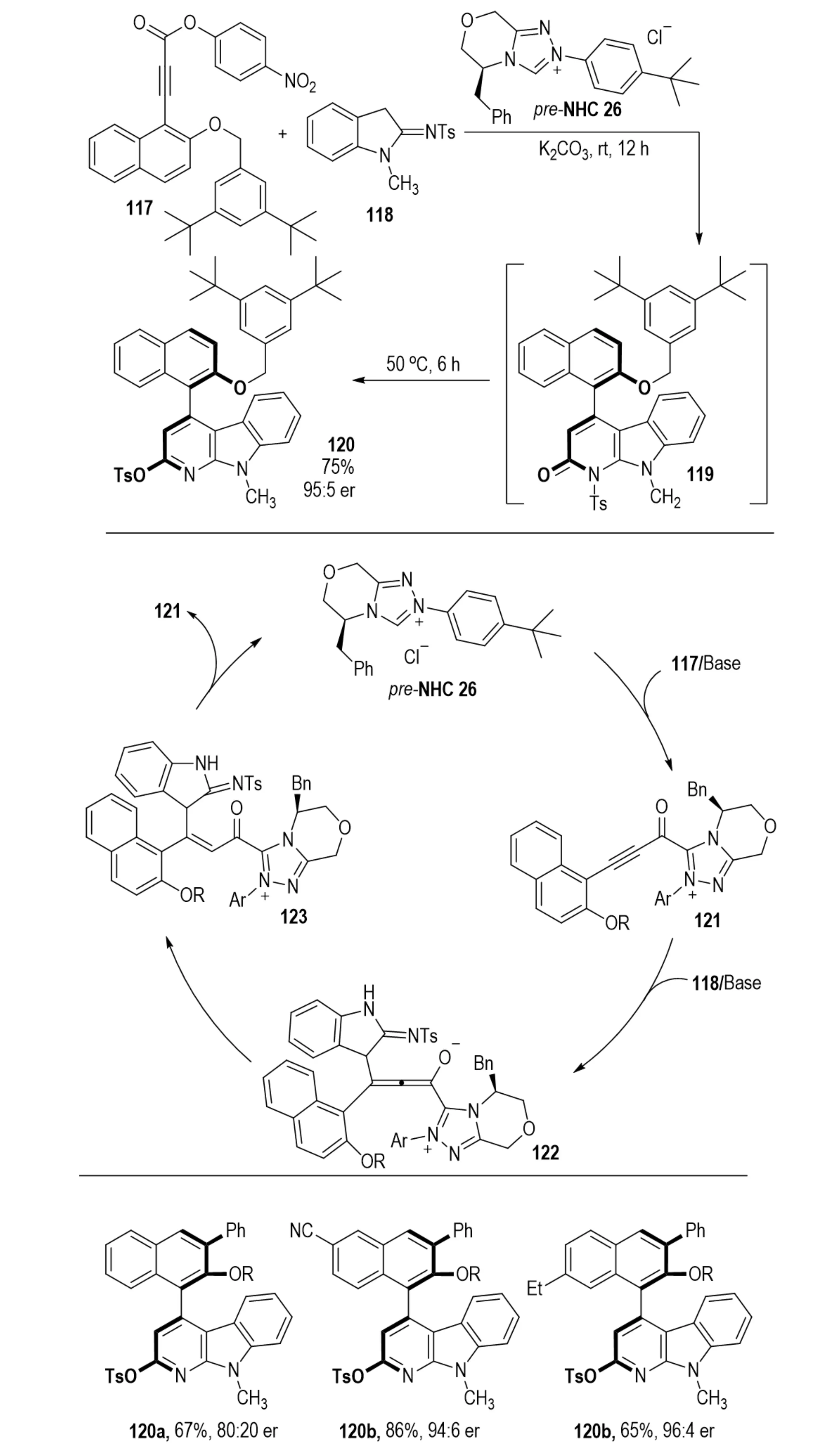
Scheme 23. NHC-catalyzed atroposelective synthesis of axially chiral 4-aryl α-carbolines via formal [3+3] annulation. NHC: N-heterocyclic carbene.
2.3 Construction of axially chiral compounds via Stetter reaction
In 2021, Barik et al. developed an NHC-catalyzed desymmetrization strategy employing prochiral N-aryl maleimides (Scheme 24)[49]. This protocol enabled the highly enantioselective synthesis of axially chiral N-aryl succinimides 126 via an intermolecular Stetter–aldol cascade between dialdehydes 124 and N-aryl maleimides 125, followed by oxidation. Mechanistic investigations, supported by DFT calculations, indicated a pathway involving Breslow intermediate formation between the NHC and dialdehyde, enantioselective conjugate addition to the maleimide, intramolecular aldol reaction, and subsequent oxidation. Both experimental and computational analyses confirmed that the tert-butyl substituent on the N-aryl ring enforces axial chirality by introducing a high rotational barrier (32.4-33.9 kcal·mol-1) along the C–N bond, which is essential for configurational stability. At elevated temperatures, this restriction weakens, leading to reduced enantioselectivity.
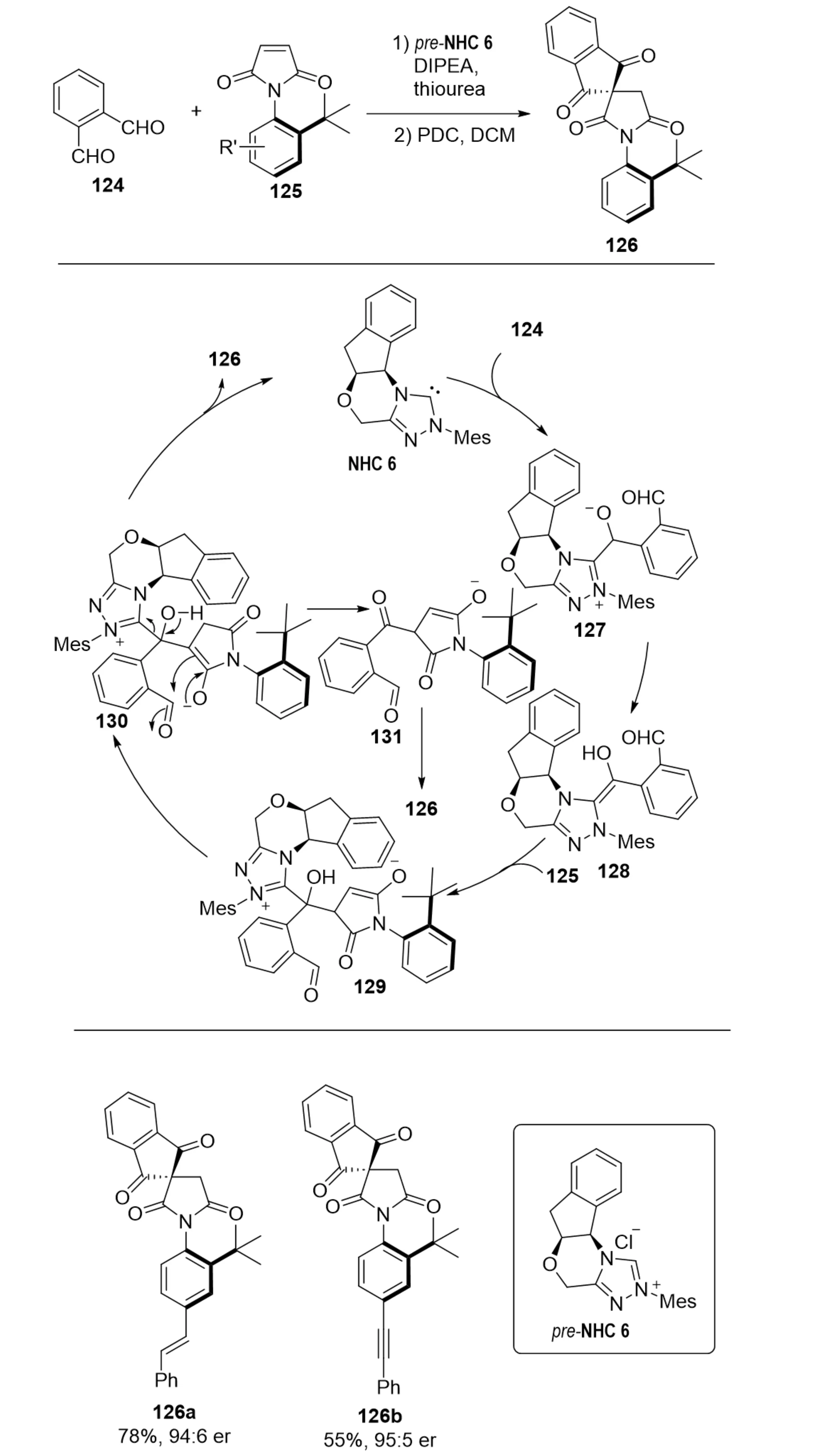
Scheme 24. NHC-catalyzed atroposelective synthesis of C–N axially chiral succinimides via a desymmetrization strategy. NHC: N-heterocyclic carbene.
2.4 Construction of axially chiral compounds via activation of imine intermediates
2.4.1 Formation of chiral nitriles via asymmetric N-S bond dissociation
Conventional strategies for the synthesis of axially chiral benzonitriles have primarily focused on constructing C(sp2)–C(sp2) stereogenic axes to induce axial chirality[50]. In 2022, Lv et al. reported an NHC-catalyzed DKR strategy for the efficient synthesis of axially chiral benzonitriles 133 (Scheme 25)[51]. In this approach, racemic 2-arylbenzaldehydes 132 react with sulfonamides under NHC catalysis to directly generate chiral benzonitriles, establishing a novel route to this class of scaffolds. The enantioselectivity is controlled by three key factors: (1) dissociation of the p-toluenesulfonate group, identified by DFT calculations as both the rate- and stereodetermining step, dictating the formation of the stereogenic axis; (2) the 2-hydroxy substituent of the substrate stabilizes transition states through hydrogen bonding with the imine moiety of the aza-Breslow intermediate, lowering the activation barrier and enhancing stereocontrol; (3) covalent and non-covalent interactions between the NHC catalyst and substrate synergistically regulate the DKR process, collectively ensuring high enantioselectivity.
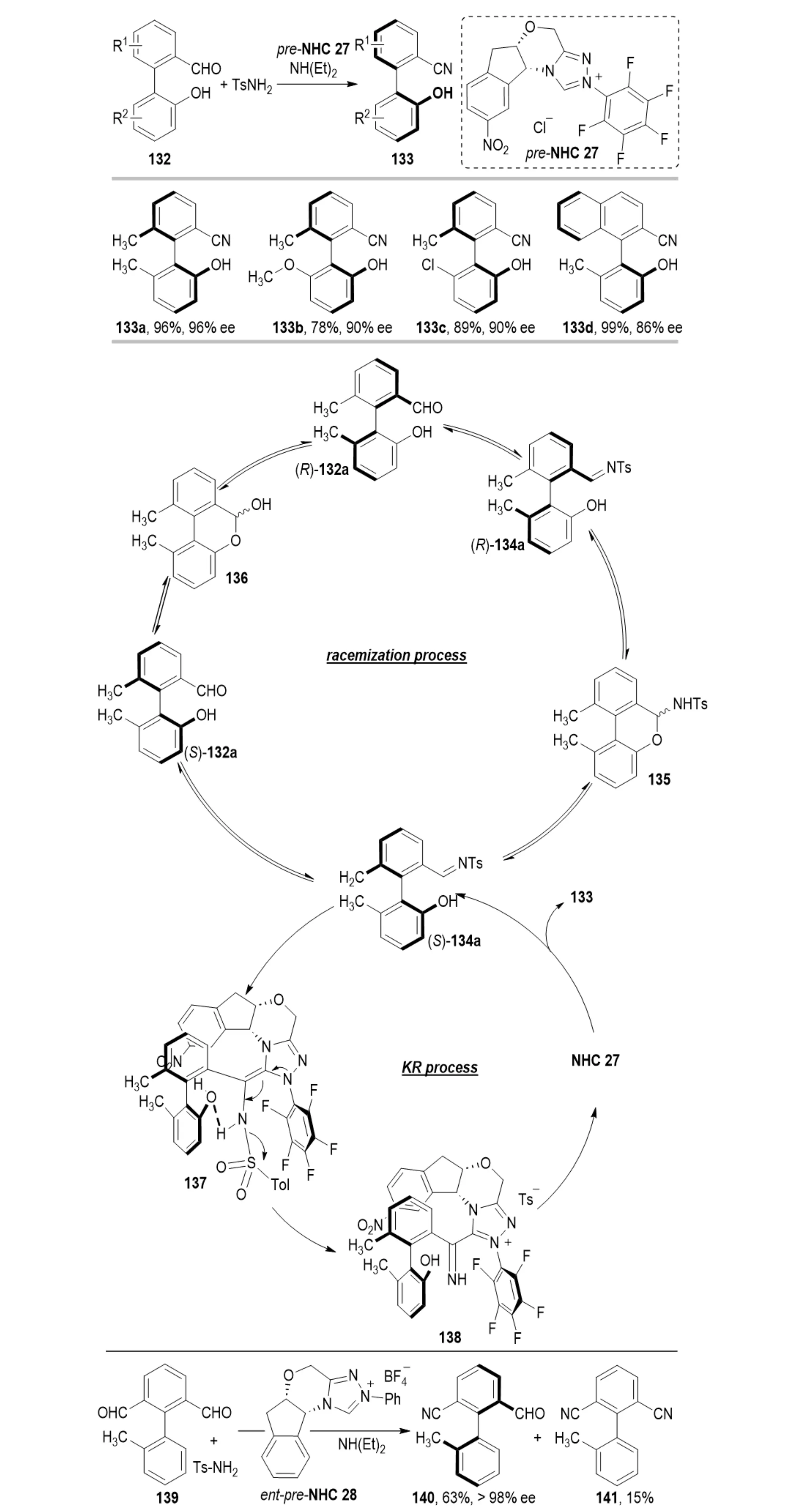
Scheme 25. NHC-catalyzed atroposelective synthesis of axially chiral benzonitriles and aryl monoaldehydes via dynamic processes. NHC: N-heterocyclic carbene.
In 2024, Cai et al. reported an NHC-catalyzed strategy for the synthesis of chiral aryl monoaldehydes 140 via nitrile formation and desymmetrization[52]. In this system, symmetric biaryl dialdehydes 139 react with p-toluenesulfinate to furnish aryl monoaldehydes featuring both aldehyde and nitrile groups. The process proceeds through a dual mechanism involving kinetic resolution and dynamic desymmetrization.
2.4.2 Construction of axially chiral compounds via aza-acylation reaction
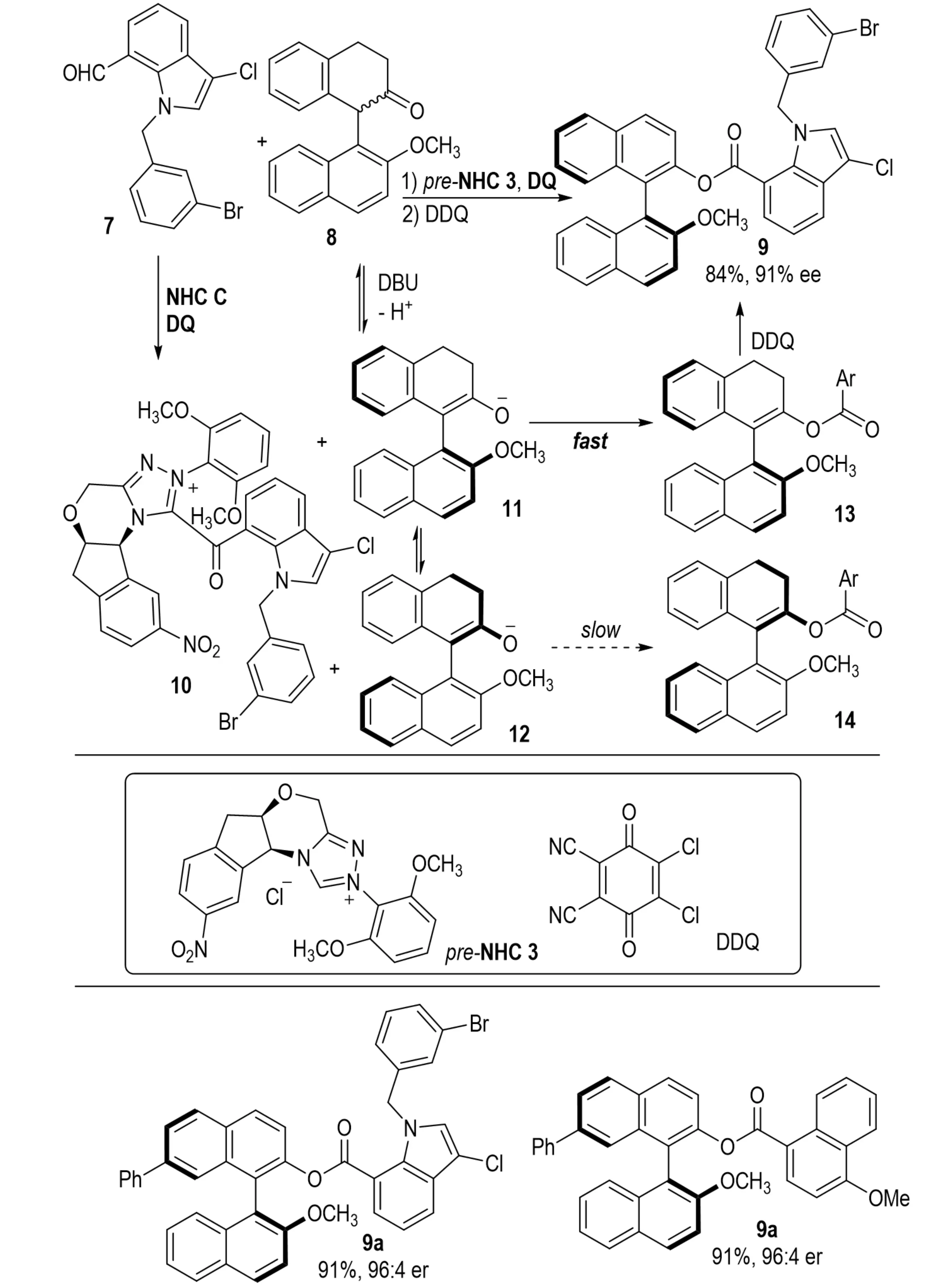
Axially chiral bridged biaryl compounds hold considerable value in natural products and as chiral ligands or catalysts[53]. In 2023, Yang et al. reported an NHC-catalyzed imine activation strategy under oxidative conditions for the construction of axially chiral bridged biaryls containing seven-membered 1,3-oxazepine rings 144 (Scheme 26)[54]. The transformation proceeds via intramolecular desymmetrization and exhibits high stereoselectivity, which is governed by three critical factors: (1) favorable frontier molecular orbital and non-covalent interactions in the dominant transition state; (2) steric effects of the 6-position substituent on the benzene ring, where bulky groups restrict axial rotation and stabilize the chiral axis, while hydrogen substitution fails to produce resolvable products; (3) structural features of the NHC catalyst, where electron-deficient substituents enhance enantiocontrol through specific substrate–catalyst interactions.
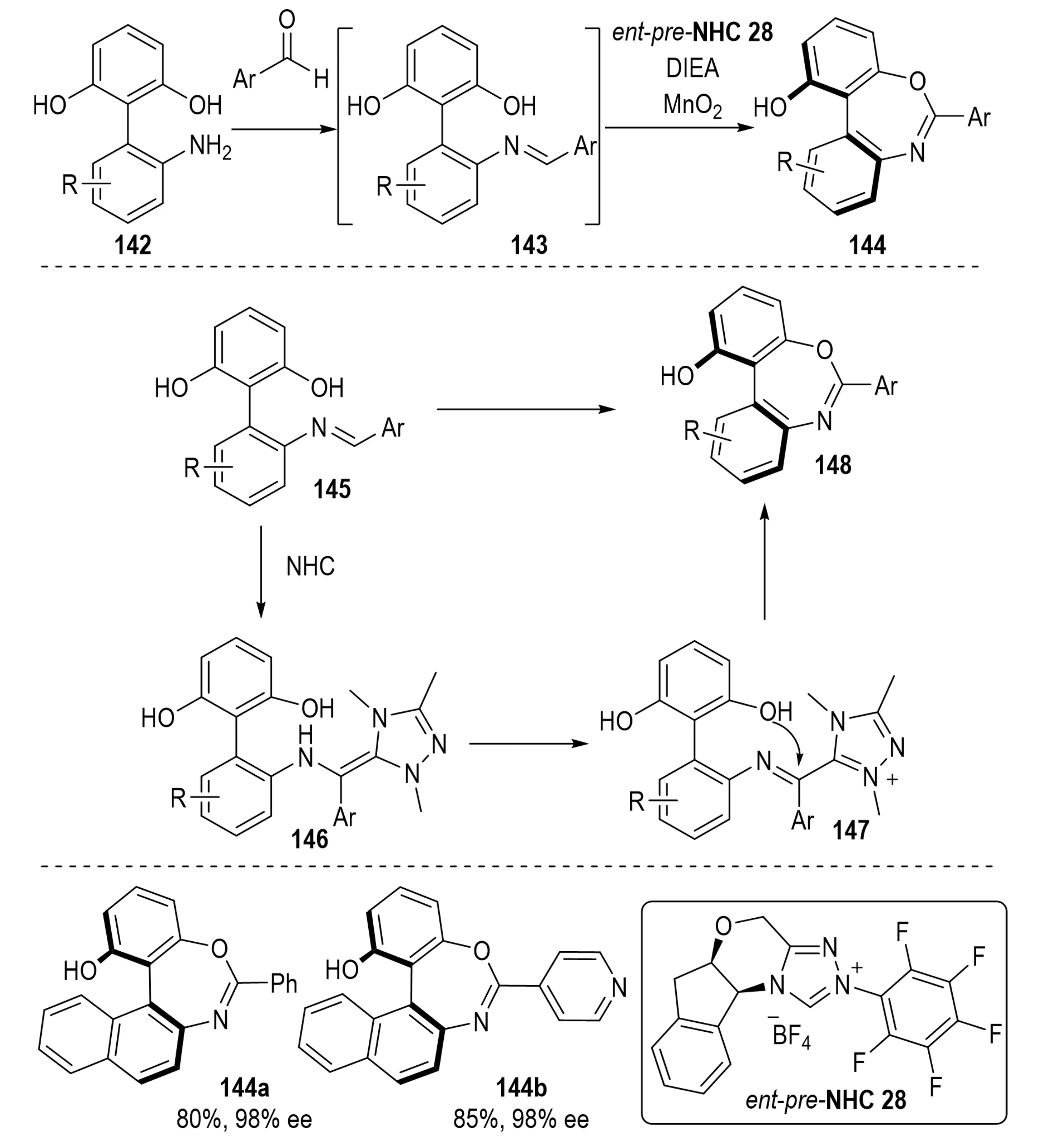
Scheme 26. NHC-catalyzed atroposelective synthesis of bridged biaryls via imine activation and intramolecular desymmetrization. NHC: N-heterocyclic carbene.
2.5 Atroposelective cycloaddition reactions catalyzed by NHC activated via enolate
In 2019, Lu et al. reported a diastereo- and atroposelective synthesis of bridged biaryls featuring an eight-membered lactone bridge 150 (Scheme 27)[55]. Their strategy employs an NHC-catalyzed cascade reaction between propargylic alcohols 149 and α,β-unsaturated aldehydes. The catalytic cycle initiates with propargylic substitution by an azolium enolate to generate an allene intermediate, which subsequently undergoes bidirectional cyclization to afford bridged biaryl products incorporating benzofuran or indole motifs. This protocol exhibits diverse substrate scope, tolerating a wide range of benzofuran- and indole-derived substrates, and enables stereoconvergent reactions with unsymmetrical propargylic alcohols. The resulting products feature both stable axial and central chirality, and cleavage of the lactone bridge further provides access to novel axially chiral biaryls.
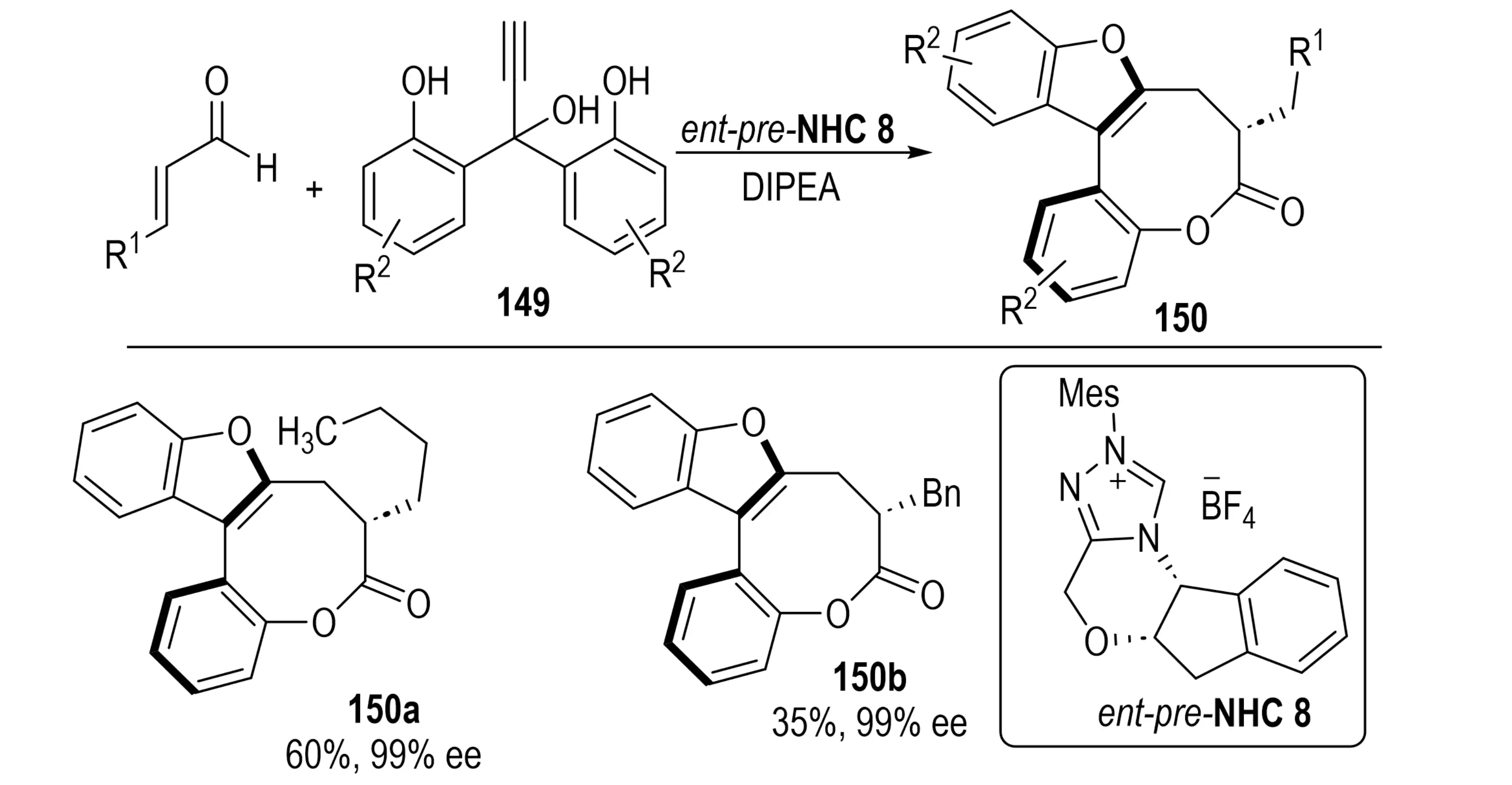
Scheme 27. NHC-catalyzed diastereo- and atroposelective synthesis of bridged biaryls with eight-membered lactone bridges via bidirectional cyclization. NHC: N-heterocyclic carbene.
α-Carbolinone derivatives have attracted significant interest due to their diverse biological activities, including anti-anxiety, anti-cancer, and anti-fungal properties[56]. In 2023, Wang et al. reported an NHC-catalyzed cascade strategy for the asymmetric synthesis of axially chiral α-carbolinones (Scheme 28)[57]. This method utilizes α,β-unsaturated iminoindole derivatives 152 and α-chloroaldehydes 151 as substrates. The reaction commences with an NHC-catalyzed [4+2] annulation to furnish dihydrocarbolinones bearing central chirality, which are subsequently oxidized with DDQ to induce central-to-axial chirality transfer, affording 3,4-disubstituted α-carbolinones 153. The high enantioselectivity is attributed to the combined influence of the steric and electronic features of the chiral NHC catalyst, the intrinsic diastereoselectivity of the [4+2] annulation, and substituent effects of the substrates.
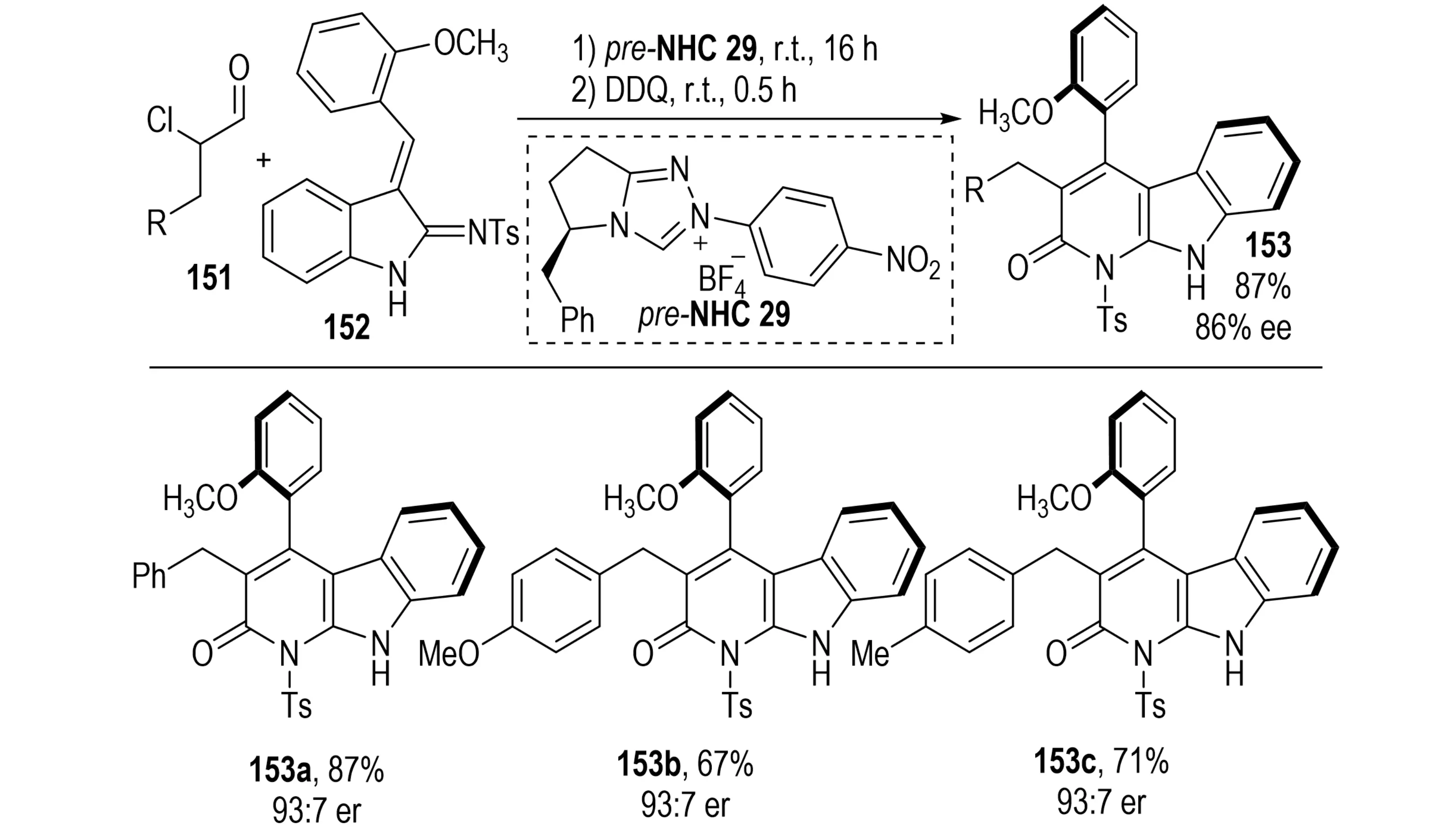
Scheme 28. NHC-catalyzed NHC-Catalyzed Atroposelective Synthesis of Axially Chiral α-Carbolinones via Central-to-Axial Chirality Conversion. NHC: N-heterocyclic carbene.
3. NHC-Catalyzed Asymmetric Synthesis of Planar Chiral Compounds
3.1 Construction of planar chiral compounds via acylation reactions
Planar chiral ferrocene derivatives have garnered extensive applications in asymmetric catalysis, medicinal chemistry, and functional materials[58]. In 2022, Lv et al. reported an NHC-catalyzed enantioselective desymmetrization for the synthesis of planar chiral ferrocenes, providing a mild and transition-metal-free route to these scaffolds (Scheme 29)[59]. In this method, ferrocene dicarbaldehydes 154 bearing prochiral planes serve as substrates. Selective activation of one aldehyde group under chiral NHC catalysis, followed by oxidation, generates an acylazolium intermediate. Subsequent esterification or thioesterification with phenols or thiols affords desymmetrized planar chiral ferrocenes 155. The enantioselectivity arises from the steric and electronic features of the NHC catalyst, fine-tuning of reaction conditions, and substrate substituent effects. This work represents the first application of NHC-catalyzed desymmetrization to the synthesis of planar chiral [2.2]paracyclophanes, demonstrating metal-free conditions, operational simplicity, broad substrate scope, and excellent stereoselectivity. Moreover, it elucidates the distinct enantiocontrol mechanisms between pseudo-ortho and pseudo-geminal diformyl derivatives. The demonstrated gram-scale feasibility and potential for diversified derivatization provide an innovative platform for the development of asymmetric catalytic ligands and functional materials, thereby advancing the field of planar chiral molecular synthesis.
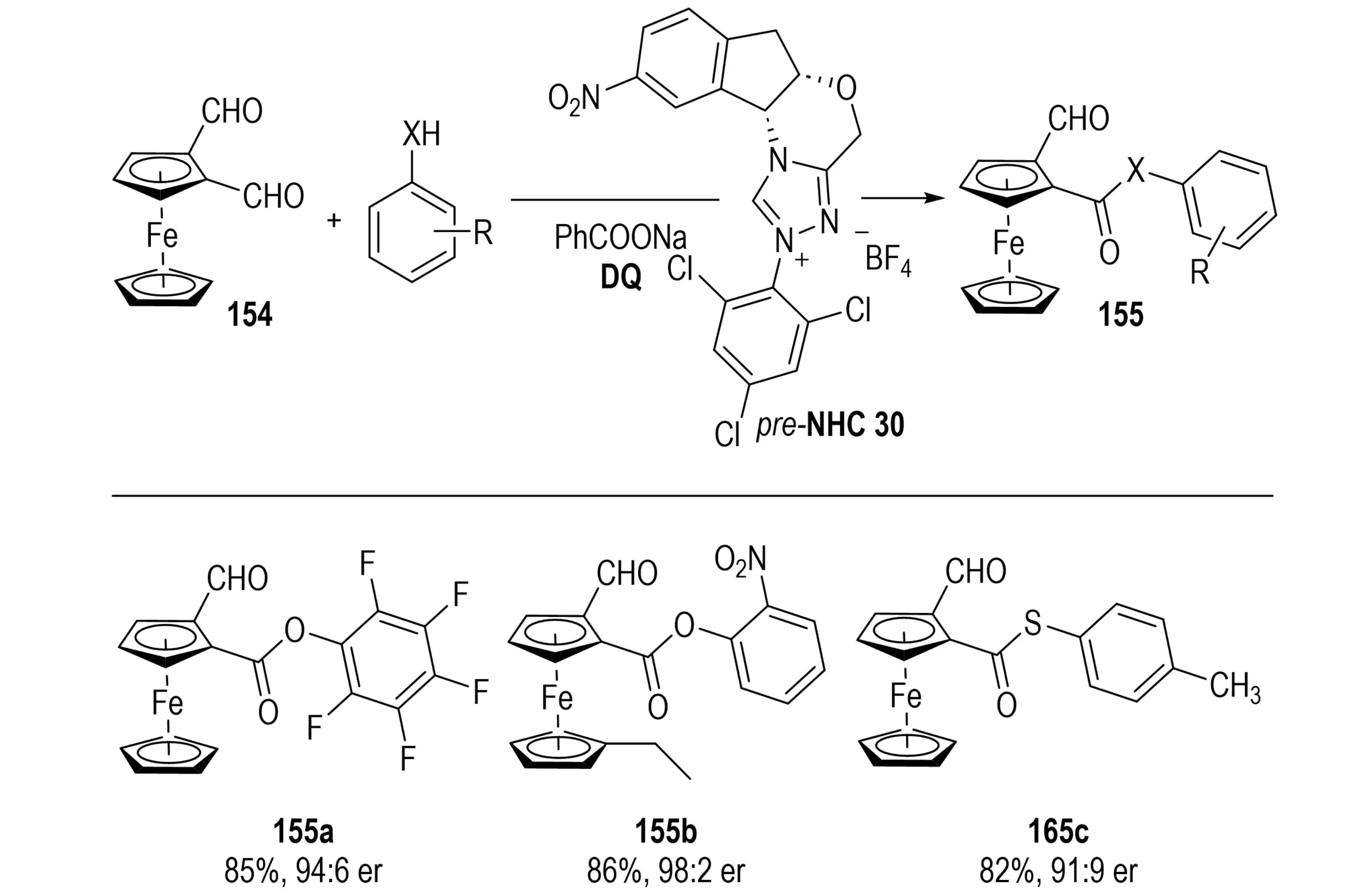
Scheme 29. NHC-catalyzed enantioselective desymmetrization of ferrocene dicarbaldehydes for synthesis of planar chiral derivatives. NHC: N-heterocyclic carbene.
[2.2]Paracyclophanes, another crucial class of planar chiral molecules, display unique chirality and conformational rigidity, enabling diverse applications in asymmetric catalysis and materials science[60]. In 2023, Dočekal et al. introduced an NHC-catalyzed enantioselective desymmetrization of prochiral diformyl[2.2]paracyclophanes 156 (Scheme 30)[61]. Using amino acid-derived NHC precursors, the transformation proceeds through selective aldehyde activation, oxidation to generate an acylazolium intermediate, and esterification or thioesterification with alcohols or thiols. Mechanistic studies revealed that in pseudo-para derivatives, enantioselectivity originates from a synergistic interplay of desymmetrization and KR, facilitated by reversible Breslow intermediate formation. In contrast, pseudo-gem derivatives achieve efficient desymmetrization through irreversible Breslow intermediate formation.
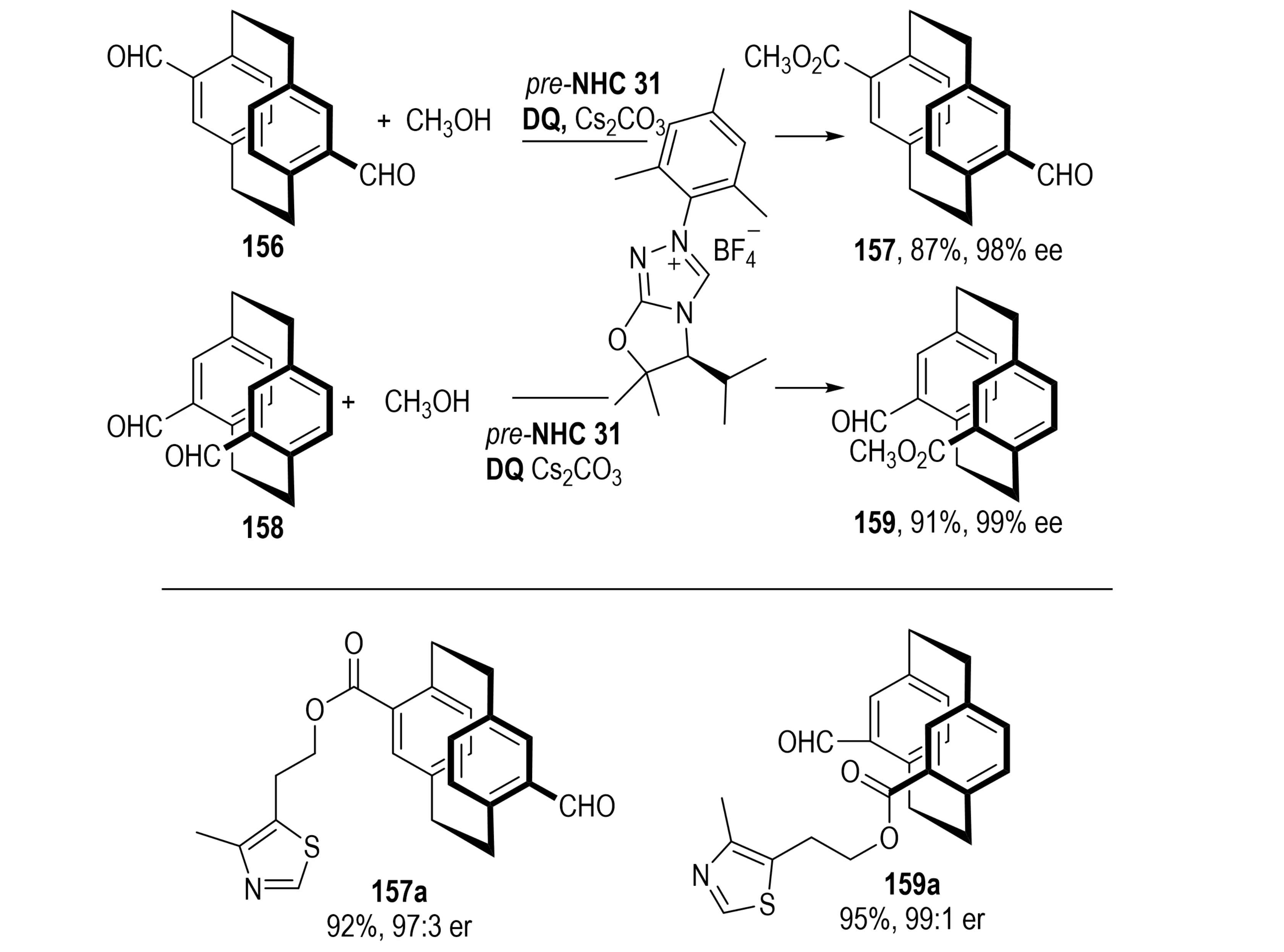
Scheme 30. NHC-catalyzed enantioselective desymmetrization of diformyl[2.2]paracyclophanes via acylazolium intermediates. NHC: N-heterocyclic carbene.
Macrolactones are prevalent motifs in natural products, pharmaceuticals, and agrochemicals, with their conformational and stereochemical features closely linked to biological function[62]. In 2024, Lv et al. disclosed an NHC-catalyzed planar-chiral macrolactonization, establishing a non-enzymatic organocatalytic route to planar chiral macrolactones from achiral bifunctional substrates (Scheme 31)[63]. The process begins with oxidation of aldehyde-containing substrates 160 to generate an acylazolium intermediate, which undergoes intramolecular hydroxyl acylation to furnish macrolactones 161. High enantioselectivity is attributed to the steric and electronic features of the catalyst pre-NHC 45. Cooperative catalysis with hydrogen-bond donor 162 further enhances selectivity via simultaneous hydrogen bonding between the thiourea moiety, the substrate hydroxyl group, and the acylazolium intermediate. Concurrently, Li et al. developed an NHC-catalyzed DKR strategy that converts racemic aldehyde-functionalized cyclophanes into planar-chiral macrolactones 164 via oxidative esterification[64]. The same authors described a highly enantioselective NHC-catalyzed macrolactonization as an efficient approach for synthesizing planar chiral macrocycles 166[65]. Additionally, in 2024, Yang et al. reported an intramolecular asymmetric macrocyclization catalyzed by NHC, enabling access to indole- and pyrrole-based planar-chiral macrocycles 168 featuring both planar and axial chirality, with excellent enantioselectivity and diastereoselectivity exceeding 19:1[66].
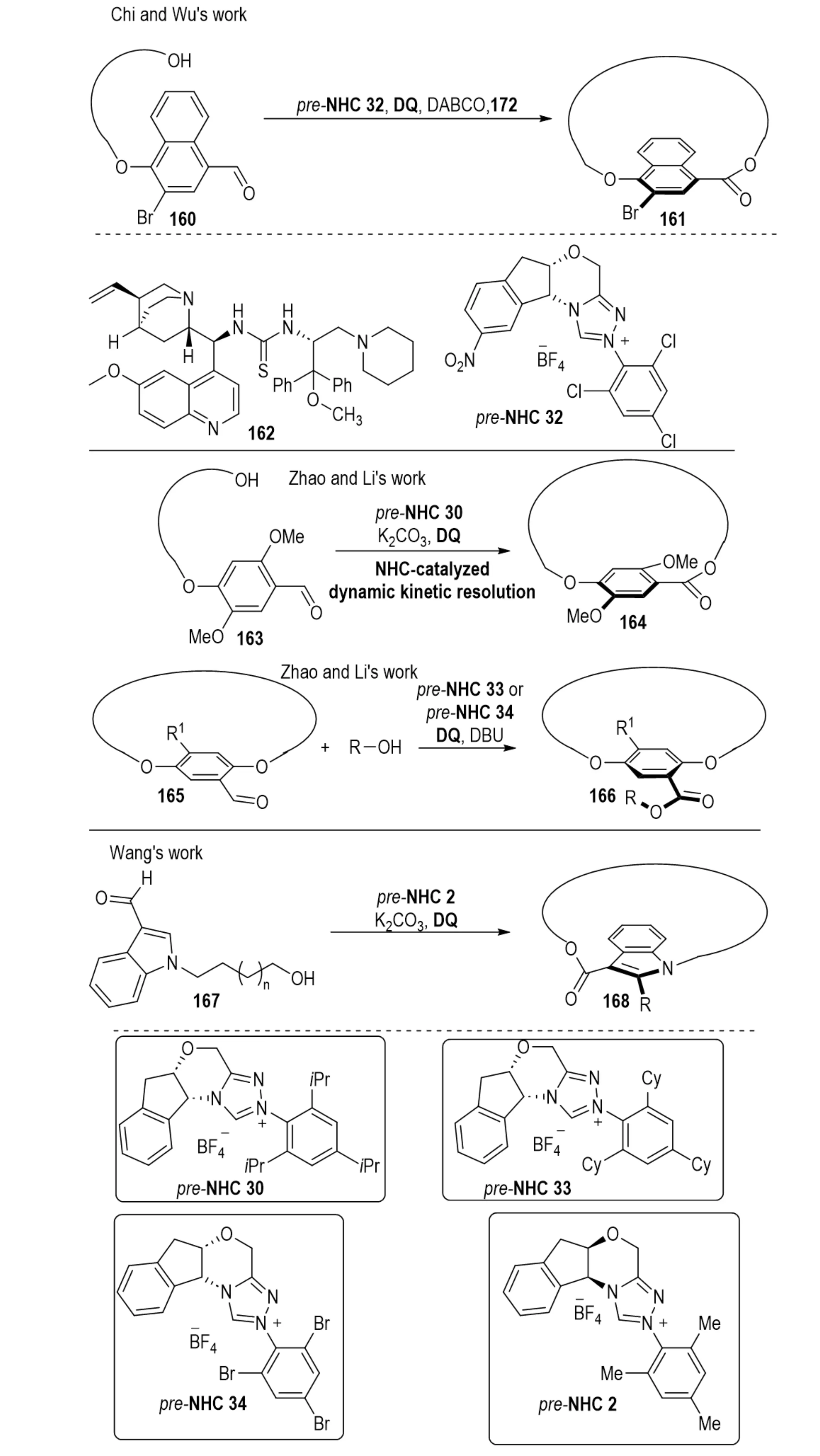
Scheme 31. NHC-catalyzed enantioselective macrolactonization strategies for constructing planar chiral macrocycles. NHC: N-heterocyclic carbene.
3.2 Construction of planar chiral compounds via conjugate addition enabled by LUMO activation
In 2024, Liu et al. reported a chemodivergent parallel kinetic resolution (PKR) strategy for the asymmetric synthesis of planar-chiral cyclophanes using a single chiral NHC catalyst (Scheme 32)[67]. In this system, two achiral esters, acetylenic ester 69 and cinnamic ester 170, were simultaneously activated to react with the enantiomers of racemic planar-chiral imine substrate 171. This process afforded products pyridine derivative 172 and lactam 173 with complementary stereoselectivities, achieving moderate yields and high optical purity. Enantiocontrol arises from the steric and electronic discrimination of racemic imine enantiomers by the bulky N-mesityl-substituted NHC catalyst. The addition of cooperative additives such as L-valinol, which provides a hydrogen bond, and HOBt, which facilitates catalyst turnover, further enhances selectivity. This method marks the first catalyst-controlled chem-divergent PKR, overcoming the limitations of traditional PKR paradigms. By employing a concise and efficient reaction design, it enables simultaneous preparation of two types of high-value planar chiral products. This method also demonstrates broad substrate scope and excellent stereoselectivity, and potential applicability in asymmetric catalysis and agrochemical development. Generally speaking, the approach not only expands the toolbox of PKR strategies, but also provides new pathways for efficient synthesis of planar chiral molecules, thereby advancing the fields of NHC catalysis and chiral resolution.
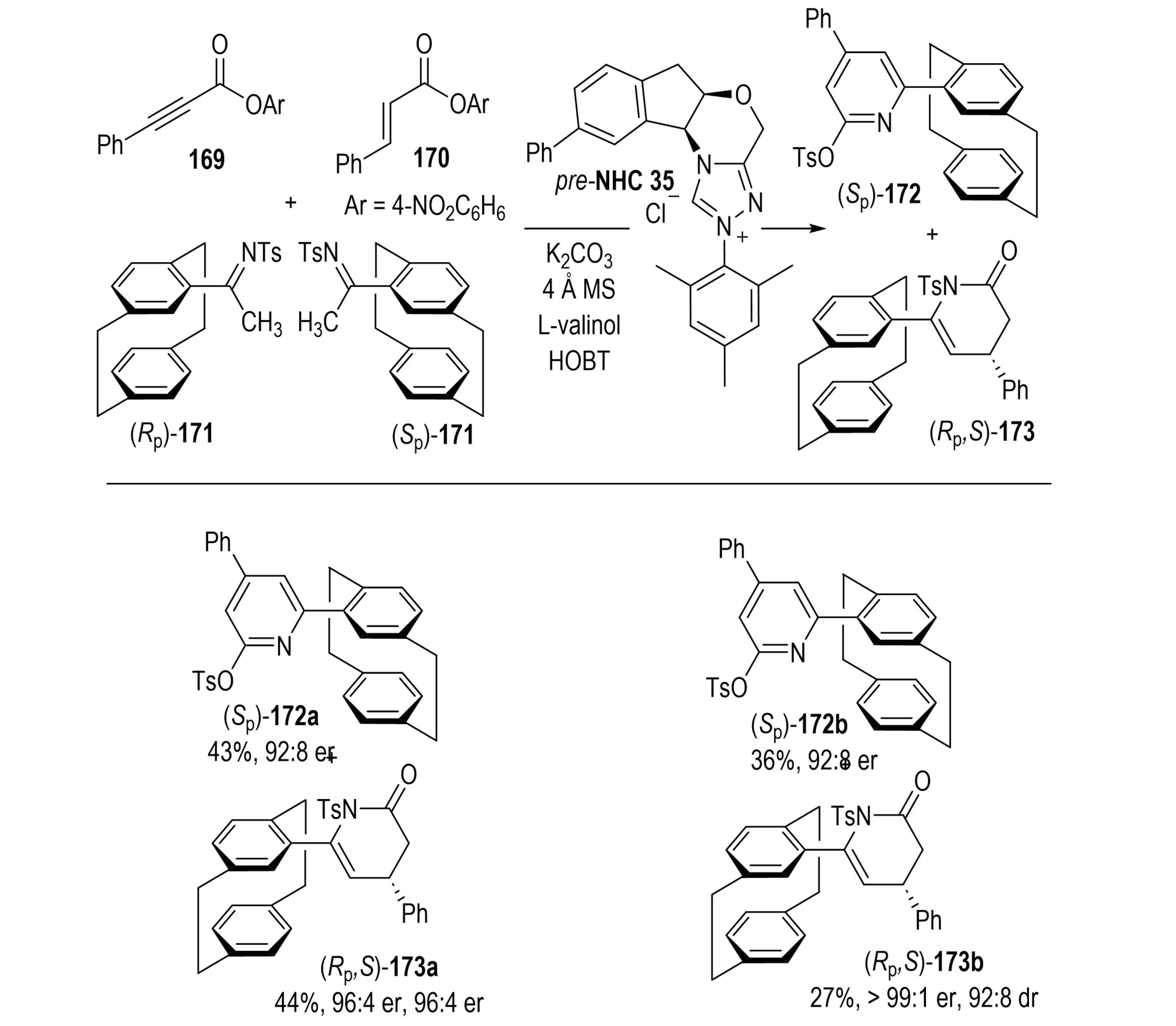
Scheme 32. NHC-catalyzed chemodivergent parallel kinetic resolution for asymmetric synthesis of planar-chiral cyclophanes. NHC: N-heterocyclic carbene.
3.3 Construction of planar chiral compounds via activation of imine intermediates
In 2024, Lv et al. reported an NHC-catalyzed asymmetric synthesis of planar chiral carbonitriles 175 via KR and desymmetrization strategies (Scheme 33)[68]. The method employs racemic [2.2]paracyclophane-derived imine substrates 174, with enantioselectivity dictated by the structural features of the chiral NHC catalyst, selective activation of one enantiomer during KR, and the steric and electronic effects of substrate substituents.
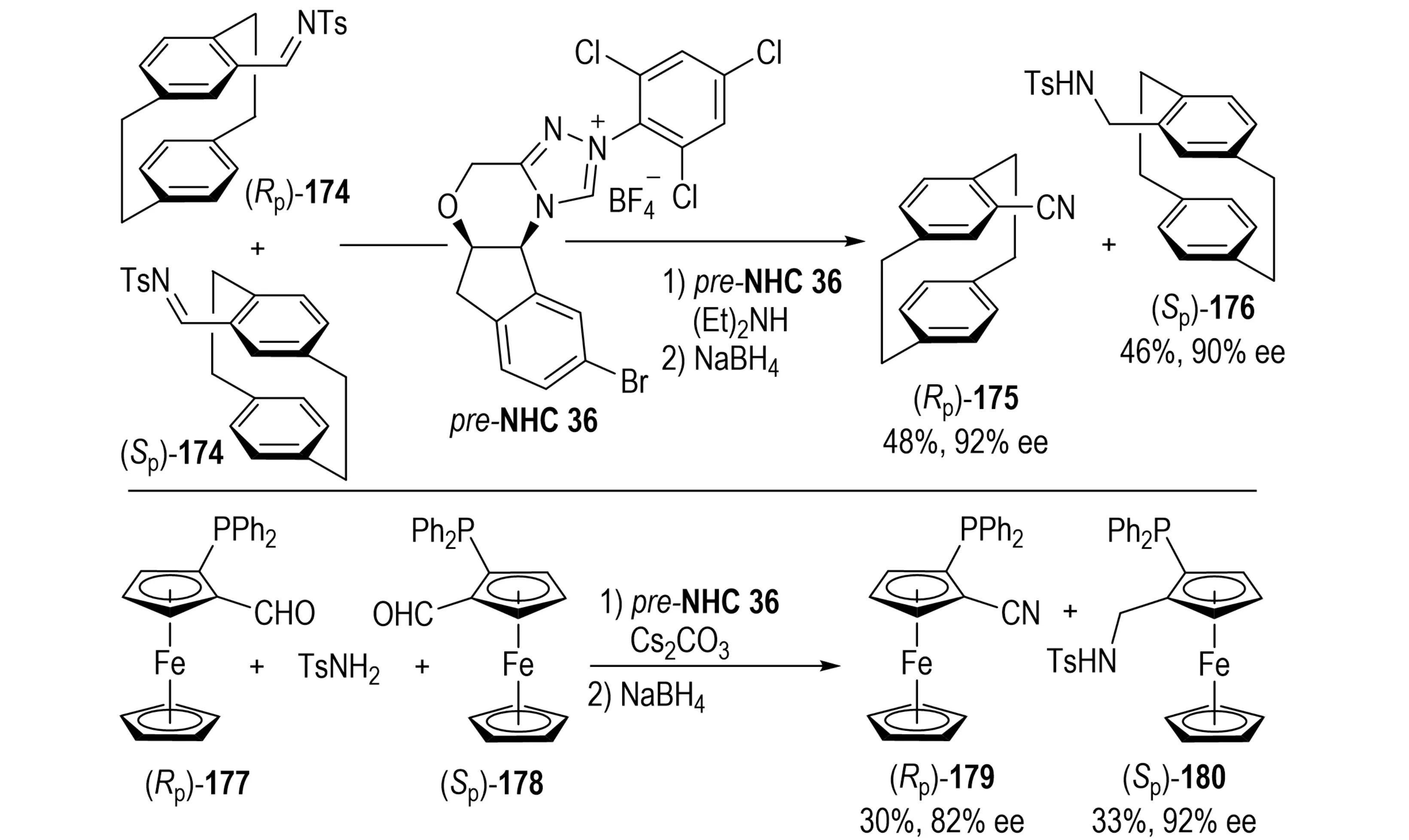
Scheme 33. NHC-catalyzed asymmetric synthesis of planar chiral carbonitriles via kinetic resolution or desymmetrization. NHC: N-heterocyclic carbene.
4. Asymmetric Synthesis of Inherently Chiral Compounds Catalyzed by N-Heterocyclic Carbenes
Inherent chirality originates from molecular frameworks that lack symmetry elements and adopt rigid, concave conformations, as exemplified by calix[4]arenes and tetraphenylene derivatives. The asymmetric catalytic synthesis of inherently chiral medium-ring compounds remains relatively underexplored, owing to challenges such as the limited availability of suitable precursors, the necessity to overcome transannular interactions and entropic barriers, and the difficulty of achieving precise enantioselective control[69].
In 2024, Shi et al. reported a notable advancement in this area through an NHC-catalyzed asymmetric formal [5+3] annulation of 1-(2-indolyl)naphthalen-2-ols 182 with ynals (Scheme 34)[70]. The catalytic system, comprising an NHC pre-catalyst, a base, a Lewis acid, and an oxidant, promoted the formation of both C–C and C–O bonds, affording a series of saddle-shaped eight-membered lactones 182 with inherent chirality.
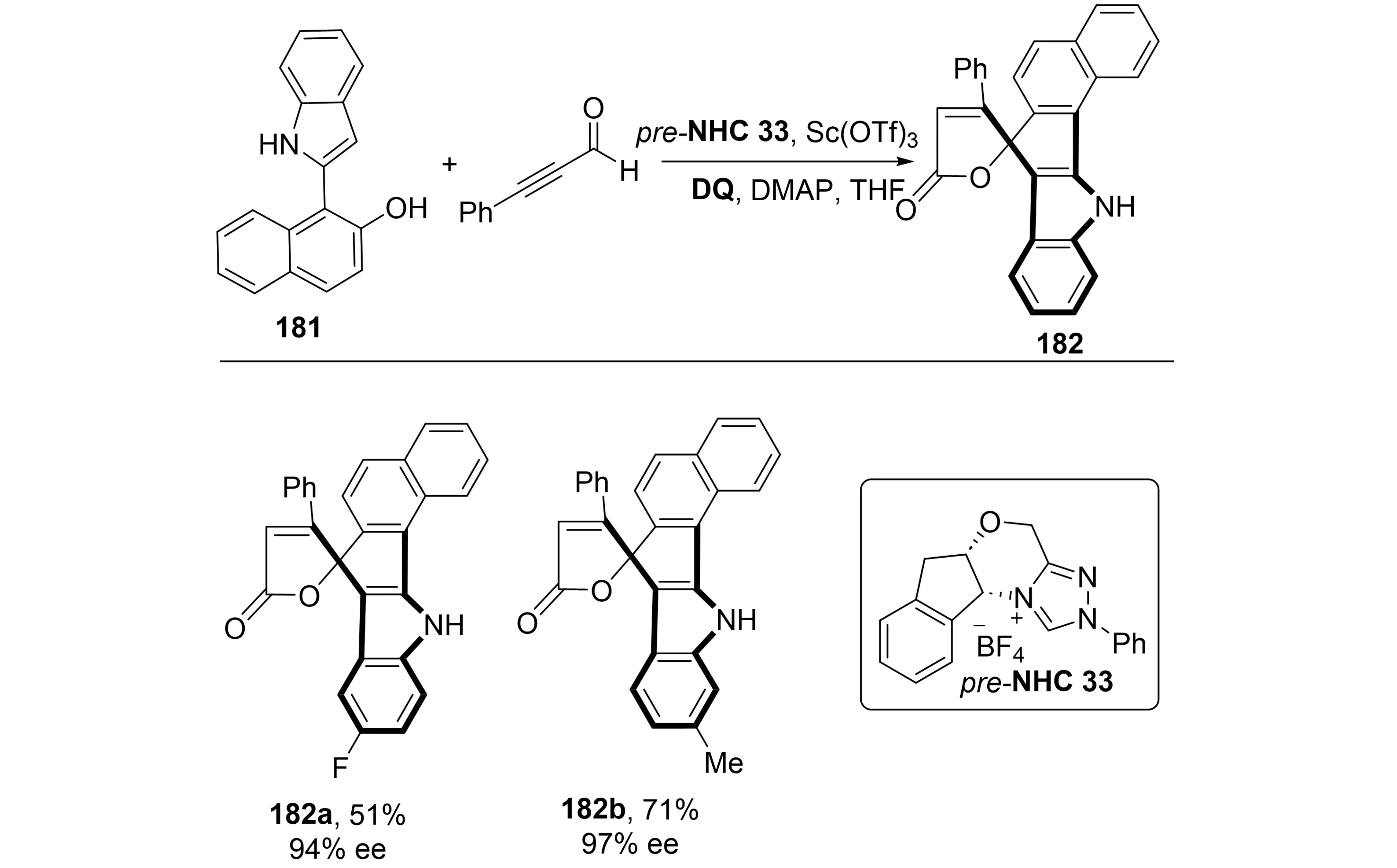
Scheme 34. NHC-catalyzed asymmetric synthesis of inherently chiral saddle-shaped lactones via formal [5+3] annulation. NHC: N-heterocyclic carbene; DQ: 3,3',5,5'-tetra-tert-butyldiphenoquinone; DMAP: 4-dimethylaminopyridine; THF: tetrahydrofuran.
Calix[4]arenes constitute a prominent class of macrocyclic compounds that exhibit inherent chirality and hold broad potential in synthetic chemistry, medicinal chemistry, and materials science[71]. However, their enantioselective synthesis has been restricted by the lack of efficient methods, particularly for accessing chiral calix[4]arenes with an ABCC substitution pattern[72]. Conventional strategies relying on racemate resolution and chiral auxiliaries, often suffer from low yields and narrow substrate scope. In 2025, Dočekal et al. addressed this limitation by developing a desymmetrization strategy employing bifunctional chiral NHC catalysis (Scheme 35)[73]. Prochiral diformylcalix[4]arenes 183 served as substrates, undergoing NHC-catalyzed asymmetric oxidative esterification to deliver ABCC-substituted calix[4]arenes in yields of up to 84% with excellent enantioselectivity. The practicality of this method was further demonstrated on gram scale, affording the product in 79% yield with 99:1 er. This metal-free method combines operational simplicity with outstanding stereoselectivity, representing the first application of NHC-catalyzed desymmetrization for the synthesis of inherently chiral calix[4]arenes. It successfully overcomes the syntheticchallenges associated with the ABCC substitution patterns.
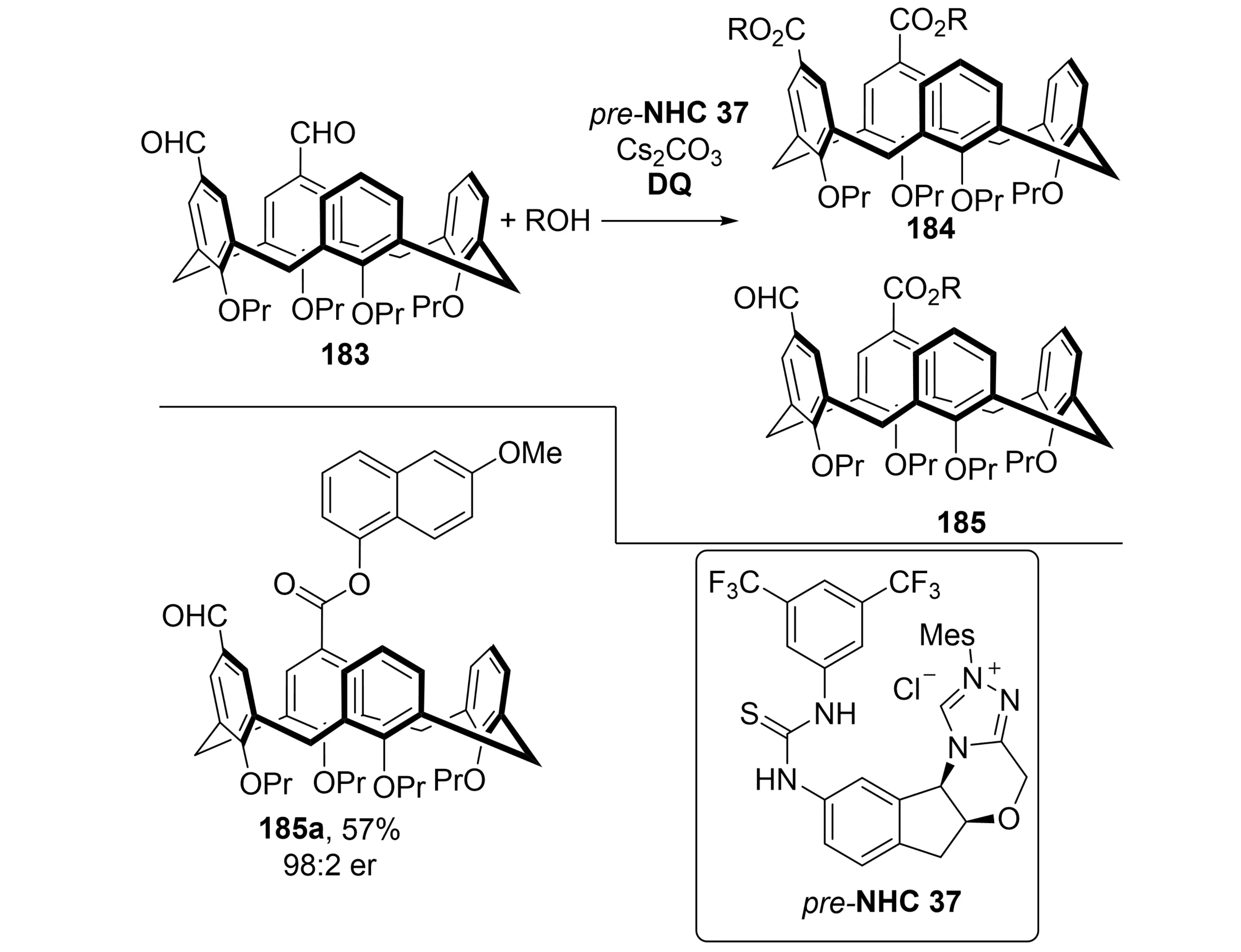
Scheme 35. NHC-catalyzed enantioselective desymmetrization of diformylcalix[4]arenes via oxidative esterification to abcc-type inherently chiral macrocycles. NHC: N-heterocyclic carbene; DQ: 4,4'-diphenoquinone.
In 2025, Wei et al. reported a carbene-catalyzed, base-controlled enantiodivergent synthesis of inherently chiral saddle-shaped eight-membered lactones (Scheme 36)[74]. This strategy employs triaryl-substituted aldehyde precursors 186, which undergo intramolecular esterification. Remarkably, the same chiral NHC catalyst, or catalysts derived from the same scaffold, can deliver either enantiomer of the product simply by adjusting the base additive. This method allows efficient access to both enantiomers of inherently chiral saddle-shaped eight-membered lactones without altering the chiral catalyst, simply by adjusting the base, achieving a balance between synthetic economy and practical applicability. The approach provides new avenues for the synthesis of medium-ring chiral molecules and the development of agricultural antimicrobial agents.
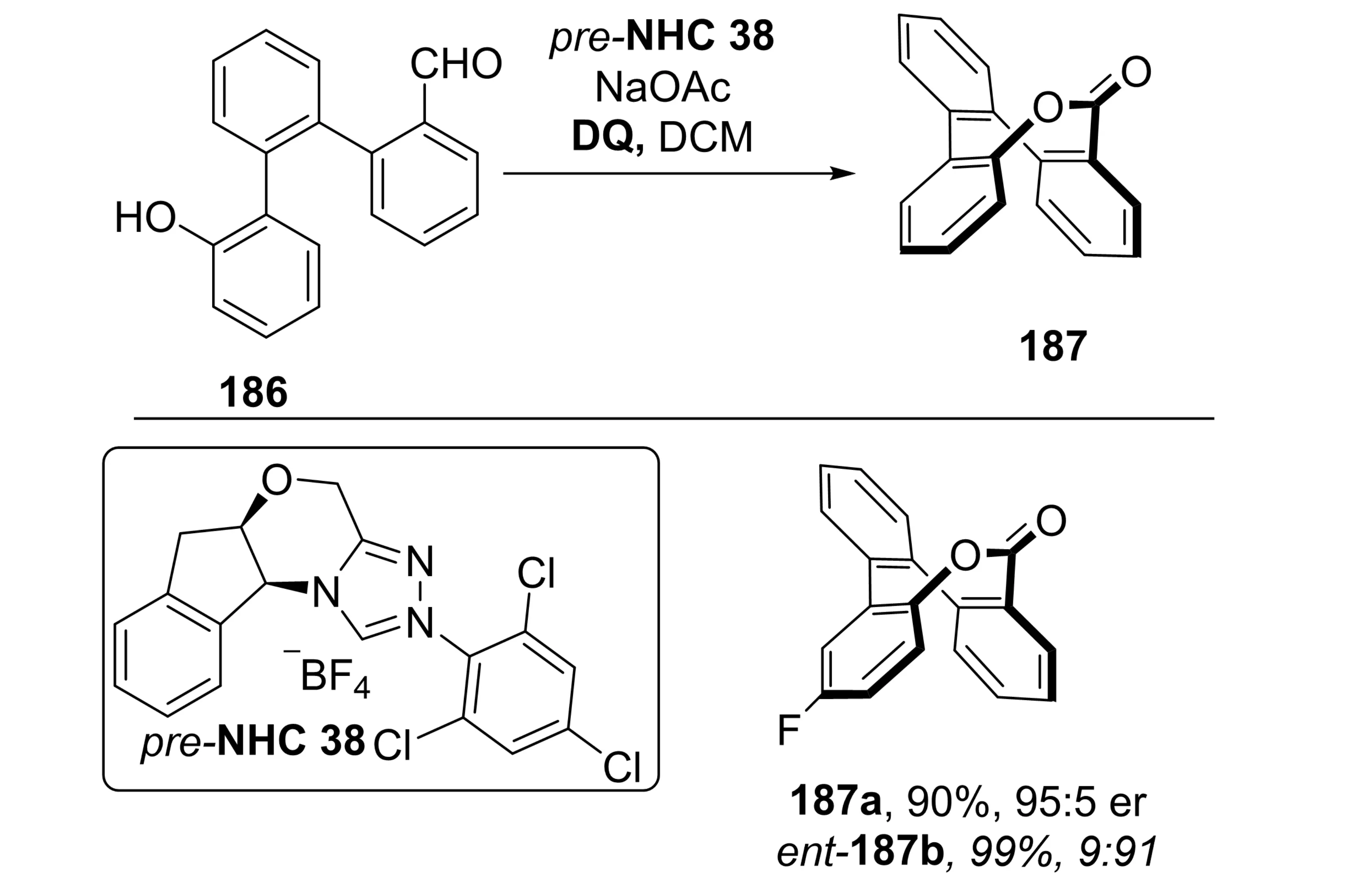
Scheme 36. Base-controlled enantiodivergent nhc catalysis for synthesis of inherently chiral saddle-shaped lactones. NHC: N-heterocyclic carbene; DQ: 4,4'-diphenoquinone; DCM: dichloromethane.
5. NHC-Catalyzed Asymmetric Synthesis of Helically Chiral Compounds
Helically chiral compounds possess remarkable photophysical and chiroptical properties, rendering them highly attractive for applications in asymmetric catalysis and functional materials[75]. In 2025, Li et al. established an N-heterocyclic carbene-catalyzed (3 + 3) annulation strategy using ynoates and enolizable polycyclic ketones, achieving efficient access to a series of pyran-2-one-fused [5]-helicenes 201 and [4]helicenes 203 with high enantioselectivity (Scheme 37)[76]. The resulting helicene derivatives were obtained in good to excellent yields and enantioselectivities and exhibited outstanding configurational stability, as evidenced by thermal racemization studies and supported by DFT calculations. This work represents the first application of NHC catalysis for the enantioselective synthesis of helical enoids, overcoming long-standing synthetic challenges in constructing heterocyclic helical architectures. The demonstrated superior stereocontrol, broad substrate compatibility, and remarkable optoelectronic properties of these compounds provide novel molecular tools for applications in functional materials and chiral recognition, thereby advancing the field of organocatalytic helical chiral synthesis.
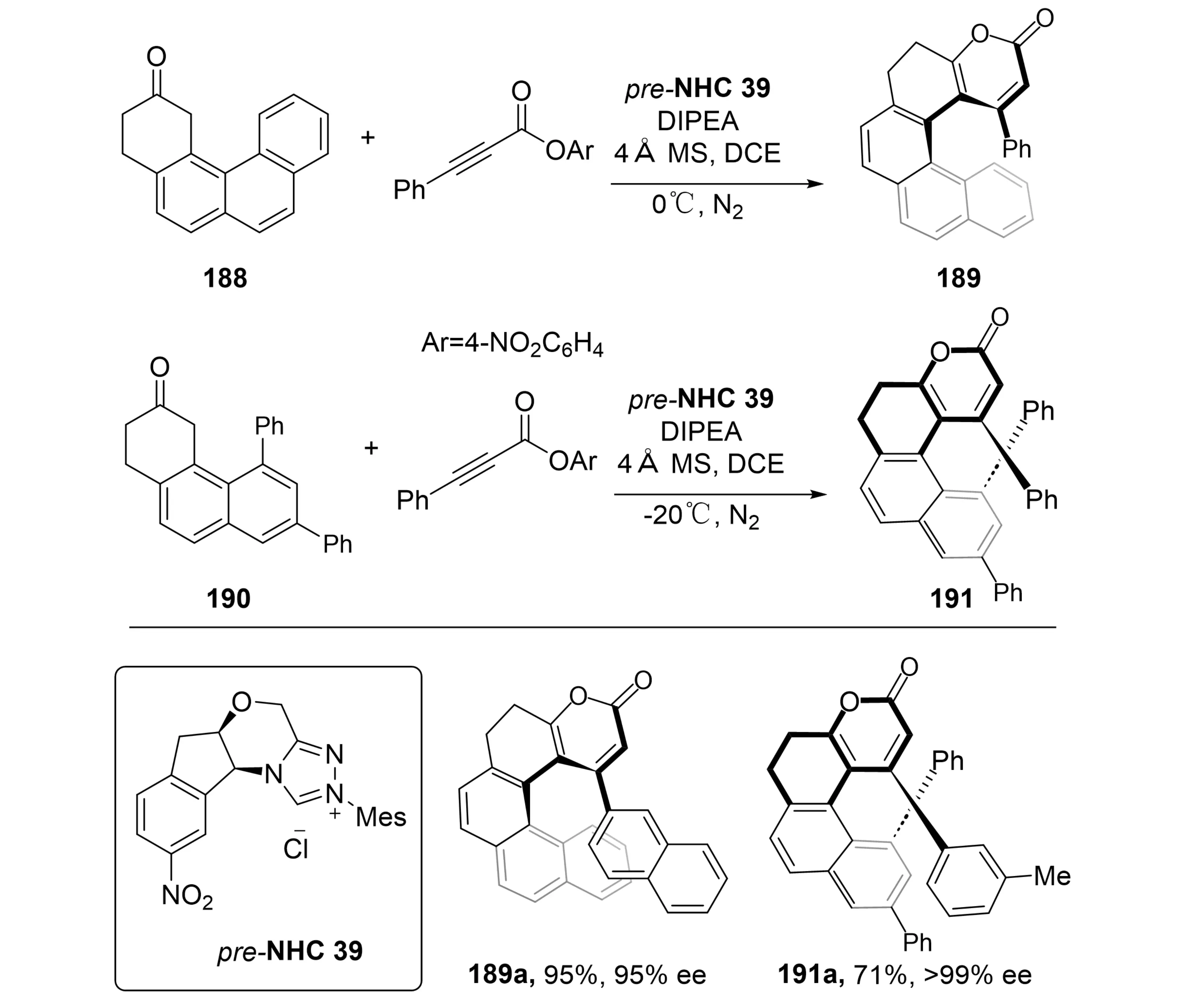
Scheme 37. NHC-catalyzed asymmetric synthesis of helically chiral compounds. NHC: N-heterocyclic carbene; DIPEA: N,N-diisopropylethylamine; MS: molecular sieves; DCE: 1,2-dichloroethane.
6. Summary and Outlook
NHC organocatalysis has been developed as a powerful strategy for constructing complex molecules, particularly those featuring stereogenic elements. While the field was initially dominated by the formation of central chirality, recent years have witnessed groundbreaking advances in applying NHCs to the asymmetric synthesis of non-central chiral compounds, specifically, axially chiral, planar chiral, inherently chiral, and spirocyclic molecules. These advances have significantly expanded the scope of NHC catalysis.
This review systematically summarizes the key developments in this emerging frontier. The core strategy therein leverages the unique catalytic modes of NHCs to generate highly reactive intermediates, such as Breslow intermediates, acylazoliums, and homoenolates, from precursors like aldehydes and carboxylic acid derivatives. These intermediates are subsequently harnessed in enantioselective reactions with prochiral or racemic substrates that possess the potential for non-central chirality, effectively controlling molecular conformation around an axis, plane, or helix. KR, DKR desymmetrization, cyclization reactions and other methodologies have been successfully applied to the asymmetric synthesis of various non-central chiral molecules with significant practical value, such as atropisomeric biaryls, chiral phosphine oxides, aryl alkenes, and polycyclic helical structures. NHC catalysis not only provides efficient and atom-economical routes to these scaffolds, but is also highly valued for its mild reaction conditions and excellent stereocontrol.
Despite these remarkable achievements, the field remains in its early stages and presents abundant opportunities and challenges for future research. Future efforts are likely to focus on underexplored chiral topologies and more complex, functionalized molecular architectures beyond current mainstream scaffolds. New NHC-triggered activation pathways will be explored beyond the established umpolung and acylazolium chemistry is a key frontier. This could involve novel radical processes or the activation of less conventional substrate classes. In-depth mechanistic studies, supported by computational chemistry, are crucial for understanding the origins of stereocontrol in these complex transformations and guiding the rational design of more selective and powerful catalysts. Furthermore, integrating NHC catalysis with other catalytic modes, such as transition metal catalysis, photoredox catalysis, or Lewis acid catalysis, could unlock unprecedented tandem reactions and provide synergistic solutions for constructing challenging non-central chiral systems.
In conclusion, NHC catalysis has paved a versatile and powerful platform for accessing non-central chirality. Continued innovation in this area is poised to expand its role as an indispensable tool for the asymmetric synthesis of these functionally important molecules.
Authors contribution
Peng Chen: Investigation, writing–original draft, visualization.
Jiang Wang: Data curation, visualization.
Lingzhu Chen: Funding acquisition, project administration, methodology, writing–review & editing
ZhiChao Jin: Conceptualization, funding acquisition, supervision, writing–review & editing.
Conflicts of interest
Zhichao Jin is an Editorial Board Member of Chiral Chemistry. The other authors declare no conflicts of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (32172459, 22371057, U23A20201); the Central Government Guides Local Science and Technology Development Fund Projects [Qiankehezhongyindi (2024)007, (2023)001]; the Program of Major Scientific and Technological, Guizhou Province [Qiankehechengguo(2024)zhongda007]; the Yongjiang Plan for Innovation and Entrepreneurship Leading Talent Project in the City of Nanning (2021005); and the Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023) at Guizhou University.
Copyright
© The Author(s) 2025.
References
-
1. Liao G, Zhou T, Yao QJ, Shi BF. Recent advances in the synthesis of axially chiral biaryls via transition metal-catalysed asymmetric C–H functionalization. Chem Commun. 2019;55(59):8514-8523.[DOI]
-
2. Jia S, Hao Y, Li Y, Lan Y. Chiral medium-sized rings beyond central chirality. Nat Rev Chem. 2025;9(9):617-633.[DOI]
-
3. Zhou H, Wang XD, Ao YF, Wang DX, Wang QQ. Inherently chiral molecular barrels via directional cascade hooping. Chin Chem Lett. 2025.[DOI]
-
4. Feng H, Lv W, Ma J, Chang W, Chen Y, Wang J. Helical structures with switchable and hierarchical chirality. Appl Phys Lett. 2020;116(19):194102.[DOI]
-
5. Jiang K, Zhu C, Yan X, Li G, Lin Z, Deng Z, et al. A stereoselective decarboxylative aromatase/cyclase directs the biosynthesis of an axially chiral biphenyl framework in fasamycin. J Am Chem Soc. 2025;147(7):5596-5601.[DOI]
-
6. Deng Y, Cai H, Jin J, Song C, Lv X, Jin Z, et al. Synthesis of planar chiral compounds containing α-amino phosphonates for antiplant virus applications against potato virus Y. J Agric Food Chem. 2024;72(21):11917-11927.[DOI]
-
7. Yang J, Liu K, Chen Y, Ye H, Hao G, Du F, et al. A supramolecular bactericidal material for preventing and treating plant-associated biofilms. Nat Commun. 2025;16(1):2627.[DOI]
-
8. Zhao HW, Jiang F, Chen S, Hu J, Xiang SH, Ding WY, et al. Organocatalytic asymmetric construction and application of axially chiral spiro-bisindoles. Angew Chem. 2025;137(12):e202422951.[DOI]
-
9. Liao G, Zhang T, Lin ZK, Shi BF. Transition metal-catalyzed enantioselective C−H functionalization via chiral transient directing group strategies. Angew Chem Int Ed. 2020;59(45):19773-19786.[DOI]
-
10. Li XK, Fu SH, Yue Y, Pang RJ, Feng J, Liu RR. C2-symmetric N-N atropisomeric diphosphines: Synthesis and application in enantioselective dearomatization of heteroaryls. Chin Chem Lett. 2025.[DOI]
-
11. (a) Koy M, Bellotti P, Das M, Glorius F. N-Heterocyclic carbenes as tunable ligands for catalytic metal surfaces. Nat Catal. 2021;4(5):352-363.[DOI](b) Wang X, Wu S, Yang R, Song H, Liu Y, Wang Q. Recent advances in combining photo- and N-heterocyclic carbene catalysis. Chem Sci. 2023;14(46):13367-13383.[DOI](c) Wang ZC, Shi SL. Induced-fit chiral N-heterocyclic carbene ligands for asymmetric catalysis. Acc Chem Res. 2025;58(13):2157-2177.[DOI](d) Yan H, Zhu Y, Yang G, Gu S. Carbene-catalytic enantioselective synthesis of chiral macrocycles. Chem Sci. 2025;16(45):21259-21268.[DOI](e) Bellotti P, Koy M, Hopkinson MN, Glorius F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat Rev Chem. 2021;5(10):711-725.[DOI](f) Zhang Y, Cai H, Gan X, Jin Z. N-heterocyclic carbene-catalyzed enantioselective (dynamic) kinetic resolutions and desymmetrizations. Sci China Chem. 2024;67(2):482-511.[DOI](g) Yan H, Zhu Y, Yang G, Gu S. Carbene-catalytic enantioselective synthesis of chiral macrocycles. Chem Sci. 2025;16(45):21259-21268.[DOI]
-
12. Yang G, Guo D, Meng D, Wang J. NHC-catalyzed atropoenantioselective synthesis of axially chiral biaryl amino alcohols via a cooperative strategy. Nat Commun. 2019;10(1):3062.[DOI]
-
13. Wu Y, Li M, Sun J, Zheng G, Zhang Q. Synthesis of axially chiral aldehydes by N-heterocyclic-carbene-catalyzed desymmetrization followed by kinetic resolution. Angew Chem Int Ed. 2022;61(14):e202117340.[DOI]
-
14. Mondal B, Chen H, Maiti R, Wang H, Cai H, Mou C, et al. Carbene-catalyzed direct O-functionalization of ketone: Atroposelective access to non-C2-symmetric binaphthyls. Org Lett. 2023;25(46):8252-8257.[DOI]
-
15. Balanna K, Barik S, Barik S, Shee S, Manoj N, Gonnade RG, et al. N-heterocyclic carbene-catalyzed atroposelective synthesis of N–N axially chiral 3-amino quinazolinones. ACS Catal. 2023;13(13):8752-8759.[DOI]
-
16. Shee S, Shree Ranganathappa S, Gadhave MS, Gogoi R, Biju AT. Enantioselective synthesis of C−O axially chiral diaryl ethers by NHC-catalyzed atroposelective desymmetrization. Angew Chem. 2023;135(52):e202311709.[DOI]
-
17. Zhou BA, Li XN, Zhang CL, Wang ZX, Ye S. Enantioselective synthesis of axially chiral diaryl ethers via NHC catalyzed desymmetrization and following resolution. Angew Chem Int Ed. 2024;63(4):e202314228.[DOI]
-
18. Li L, Ti W, Miao T, Ma J, Lin A, Chu Q, et al. Atroposelective synthesis of axially chiral diaryl ethers by N-heterocyclic-carbene-catalyzed sequentially desymmetric/kinetic resolution process. J Org Chem. 2024;89(6):4067-4073.[DOI]
-
19. Wu Y, Guan X, Zhao H, Li M, Liang T, Sun J, et al. Synthesis of axially chiral diaryl ethers via NHC-catalyzed atroposelective esterification. Chem Sci. 2024;15(12):4564-4570.[DOI]
-
20. Liu Y, Yuan L, Dai L, Zhu Q, Zhong G, Zeng X. Carbene-catalyzed atroposelective construction of chiral diaryl ethers. J Org Chem. 2024;89(11):7630-7643.[DOI]
-
21. Lu S, Poh SB, Zhao Y. Kinetic resolution of 1,1'-biaryl-2,2'-diols and amino alcohols through NHC-catalyzed atroposelective acylation. Angew Chem Int Ed. 2014;53(41):11041-11045.[DOI]
-
22. Bie J, Lang M, Wang J. Enantioselective N-heterocyclic carbene-catalyzed kinetic resolution of anilides. Org Lett. 2018;20(18):5866-5871.[DOI]
-
23. Wang ZQ, Wei LW, Wang ZQ, Yang YJ, Zhao Y, Liu S, et al. Modular synthesis of polyfunctionalized axial-chiral 2-arylpyridines via cobalt-catalyzed asymmetric [2+2+2] cycloaddition of diynes and nitriles. Chin Chem Lett. 2025.[DOI]
-
24. Lu S, Poh SB, Rong ZQ, Zhao Y. NHC-catalyzed atroposelective acylation of phenols: Access to enantiopure NOBIN analogs by desymmetrization. Org Lett. 2019;21(15):6169-6172.[DOI]
-
25. Zheng Z, Liu Q, Peng X, Jin Z, Wu J. NHC-catalyzed chemo- and enantioselective reaction between aldehydes and enals for access to axially chiral arylaldehydes. Org Lett. 2024;26(4):917-921.[DOI]
-
26. Cheng H, Fu Y, Chang Q, Zhang N, Bu M, Niu Y, et al. Synthesis, biochemical evaluation and computational simulations of new cytochrome bc1 complex inhibitors based on N-(4-aryloxyphenyl) phthalimides. Chin Chem Lett. 2018;29(12):1897-1900.[DOI]
-
27. Barik S, Ranganathappa SS, Biju AT. N-heterocyclic carbene-catalyzed atroposelective synthesis of N-aryl phthalimides and maleimides via activation of carboxylic acids. Nat Commun. 2024;15(1):5755.[DOI]
-
28. Cai Y, Zhao Y, Tang K, Zhang H, Mo X, Chen J, et al. Amide C–N bonds activation by a new variant of bifunctional N-heterocyclic carbene. Nat Commun. 2024;15(1):496.[DOI]
-
29. Wang G, Yuan G, Wei C, Zhang Y, Zhu H, Yang W, et al. Carbene-catalyzed asymmetric ring-opening reaction of biaryl lactams to access axially chiral biaryls. Chin J Chem. 2024;42(15):1734-1740.[DOI]
-
30. Koutsopoulos K, Lavrentaki V, Antoniou I, Kousaxidis A, Lefkopoulou M, Tsantili-Kakoulidou A, et al. Design synthesis and evaluation of novel aldose reductase inhibitors: The case of indolyl–sulfonyl–phenols. Bioorg Med Chem. 2020;28(15):115575.[DOI]
-
31. Jin J, Huang X, Xu J, Li T, Peng X, Zhu X, et al. Carbene-catalyzed atroposelective annulation and desymmetrization of urazoles. Org Lett. 2021;23(10):3991-3996.[DOI]
-
32. Li T, Mou C, Qi P, Peng X, Jiang S, Hao G, et al. N-heterocyclic carbene-catalyzed atroposelective annulation for access to thiazine derivatives with C−N axial chirality. Angew Chem. 2021;133(17):9448-9453.[DOI]
-
33. Chu Y, Wu M, Hu F, Zhou P, Cao Z, Hui XP. N-heterocyclic carbene-catalyzed atroposelective synthesis of pyrrolo[3,4-b]pyridines with configurationally stable C−N axial chirality. Org Lett. 2022;24(21):3884-3889.[DOI]
-
34. Xu K, Li W, Zhu S, Zhu T. Atroposelective arene formation by carbene-catalyzed formal [4+2] cycloaddition. Angew Chem. 2019;131(49):17789-17794.[DOI]
-
35. Wang G, Huang J, Zhang L, Han J, Zhang X, Huang J, et al. N-heterocyclic carbene-catalyzed atroposelective synthesis of axially chiral 5-aryl 2-pyrones from enals. Sci China Chem. 2022;65(10):1953-1961.[DOI]
-
36. Zhang L, Wu Q, Ren M, Zhang H, Zhang X, Liu J, et al. N-heterocyclic carbene-catalyzed atroposelective synthesis of 5-indo-1-yl pyran-2-ones with an N−C axis from enals. Adv Synth Catal. 2023;365(20):3467-3472.[DOI]
-
37. Zhang CL, Gao YY, Wang HY, Zhou BA, Ye S. Enantioselective synthesis of axially chiral benzothiophene/benzofuran-fused biaryls by N-heterocyclic carbene catalyzed arene formation. Angew Chem Int Ed. 2021;60(25):13918-13922.[DOI]
-
38. Barik S, Das RC, Balanna K, Biju AT. Kinetic resolution approach to the synthesis of C−N axially chiral N-aryl aminomaleimides via NHC-catalyzed [3+3] annulation. Org Lett. 2022;24(29):5456-5461.[DOI]
-
39. Yan JL, Maiti R, Ren SC, Tian W, Li T, Xu J, et al. Carbene-catalyzed atroposelective synthesis of axially chiral styrenes. Nat Commun. 2022;13(1):84.[DOI]
-
40. Li Y, Duan XY, Yang C, Wei Y, Li J, Ren X, et al. Atroposelective access to dihydropyridinones with C−N axial and point chirality via NHC-catalyzed [3+3] annulation. J Org Chem. 2023;88(15):11299-11309.[DOI]
-
41. Ranganathappa SS, Dehury BS, Singh GK, Shee S, Biju AT. Atroposelective synthesis of N−N axially chiral indoles and pyrroles via NHC-catalyzed diastereoselective (3 + 3) annulation strategy. ACS Catalysis. 2024;14(9):6965-6972.[DOI]
-
42. Zhang S, Wang X, Han LL, Li J, Liang Z, Wei D, et al. Atroposelective synthesis of triaryl α-pyranones with 1,2-diaxes by N-heterocyclic carbene organocatalysis. Angew Chem Int Ed. 2022;61(52):e202212005.[DOI]
-
43. Zhang SC, Liu S, Wang X, Wang SJ, Yang H, Li L, et al. Enantioselective access to triaryl-2-pyrones with monoaxial or contiguous C−C diaxes via oxidative NHC catalysis. ACS Catal. 2023;13(4):2565-2575.[DOI]
-
44. Wang X, Zhang S, Wang SJ, An H, Xin X, Lin H, et al. An assembly of pyrano[3,2-b]indol-2-ones via NHC-catalyzed [3+3] annulation of indolin-3-ones with ynals. Chin J Chem. 2024;42(13):1487-1492.[DOI]
-
45. Wang SJ, Wang X, Xin X, Zhang S, Yang H, Wong MW, et al. Organocatalytic diastereo- and atroposelective construction of N−N axially chiral pyrroles and indoles. Nat Commun. 2024;15(1):518.[DOI]
-
46. Liang D, Chen JR, Tan LP, He ZW, Xiao WJ. Catalytic asymmetric construction of axially and centrally chiral heterobiaryls by minisci reaction. J Am Chem Soc. 2022;144(13):6040-6049.[DOI]
-
47. Luo MP, Gu YJ, Wang SG. Photocatalytic enantioselective minisci reaction of β-carbolines and application to natural product synthesis. Chem Sci. 2023;14(2):251-256.[DOI]
-
48. Ma R, Wang X, Zhang Q, Chen L, Gao J, Feng J, et al. Atroposelective synthesis of axially chiral 4-aryl α-carbolines via N-heterocyclic carbene catalysis. Org Lett. 2021;23(11):4267-4272.[DOI]
-
49. Barik S, Shee S, Das S, Gonnade RG, Jindal G, Mukherjee S, et al. NHC-catalyzed desymmetrization of N-aryl maleimides leading to the atroposelective synthesis of N-aryl succinimides. Angew Chem. 2021;133(22):12372-12376.[DOI]
-
50. Xu H, Zhu Y, Chai X, Yang J, Dong L. Rh(III)-catalyzed direct cross-dehydrogenative coupling of aromatic nitriles with heteroarenes: Rapid access to biheteroaryl-2-carbonitriles. Green Synth Catal. 2020;1(2):167-170.[DOI]
-
51. Lv Y, Luo G, Liu Q, Jin Z, Zhang X, Chi YR. Catalytic atroposelective synthesis of axially chiral benzonitriles via chirality control during bond dissociation and CN group formation. Nat Commun. 2022;13(1):36.[DOI]
-
52. Cai Y, Lv Y, Shu L, Jin Z, Chi YR, Li T. Access to axially chiral aryl aldehydes via carbene-catalyzed nitrile formation and desymmetrization reaction. Research. 2024;7:0293.[DOI]
-
53. Wang SH, Wei SQ, Zhang Y, Zhang XM, Zhang SY, Dai KL, et al. Atroposelective synthesis of biaxial bridged eight-membered terphenyls via a Co/SPDO-catalyzed aerobic oxidative coupling/desymmetrization of phenols. Nat Commun. 2024;15(1):4591.[DOI]
-
54. Yang X, Wei L, Wu Y, Zhou L, Zhang X, Chi YR. Atroposelective access to 1,3-oxazepine-containing bridged biaryls via carbene-catalyzed desymmetrization of imines. Angew Chem Int Ed. 2023;62(1):e202211977.[DOI]
-
55. Lu S, Ong JY, Yang H, Poh SB, Liew X, Seow CSD, et al. Diastereo- and atroposelective synthesis of bridged biaryls bearing an eight-membered lactone through an organocatalytic cascade. J Am Chem Soc. 2019;141(43):17062-17067.[DOI]
-
56. Sheng T, Kong M, Wang Y, Wu H, Gu Q, Chuang AS, et al. Discovery and preliminary mechanism of 1-carbamoyl β-carbolines as new antifungal candidates. Eur J Med Chem. 2021;222:113563.[DOI]
-
57. Wang HY, Li ZC, Zhang CL, Ye S. N-heterocyclic carbene-catalyzed atroposelective synthesis of axially chiral α-carbolinones via heterocycle formation. J Org Chem. 2023;88(16):11913-11923.[DOI]
-
58. Zhou Q, Qiao L, Zhang AA, Li L, Meng TJ, Fan B, et al. Enantioselective synthesis of planar chiral ferrocenes via gold(I)-catalyzed hydroarylation of N-ferrocenyl propiolamides. Green Synth Catal. 2025;6(2):198-201.[DOI]
-
59. Lv X, Xu J, Sun C, Su F, Cai Y, Jin Z, et al. Access to planar chiral ferrocenes via N-heterocyclic carbene-catalyzed enantioselective desymmetrization reactions. ACS Catal. 2022;12(4):2706-2713.[DOI]
-
60. Yu S, Bao H, Zhang D, Yang X. Kinetic resolution of substituted amido[2.2]paracyclophanes via asymmetric electrophilic amination. Nat Commun. 2023;14(1):5239.[DOI]
-
61. Dočekal V, Koucký F, Císařová I, Veselý J. Organocatalytic desymmetrization provides access to planar chiral [2.2]paracyclophanes. Nat Commun. 2024;15(1):3090.[DOI]
-
62. Qu BL, Xiao M, He L, Shi JW, Zhang Z, Xiao WJ, et al. Enantioselective macrocyclization via catalytic metallic dipole relay. Nat Catal. 2025;8(4):368-377.[DOI]
-
63. Lv X, Su F, Long H, Lu F, Zeng Y, Liao M, et al. Carbene organic catalytic planar enantioselective macrolactonization. Nat Commun. 2024;15(1):958.[DOI]
-
64. Li J, Dong Z, Chen Y, Yang Z, Yan X, Wang M, et al. N-heterocyclic carbene-catalyzed enantioselective synthesis of planar-chiral cyclophanes via dynamic kinetic resolution. Nat Commun. 2024;15(1):2338.[DOI]
-
65. Wang J, Wang M, Wen Y, Teng P, Li C, Zhao C. N-heterocyclic carbene-catalyzed highly enantioselective macrolactonization to access planar-chiral macrocycles. Org Lett. 2024;26(5):1040-1045.[DOI]
-
66. Yang G, He Y, Wang T, Li Z, Wang J. Atroposelective synthesis of planar-chiral indoles via carbene catalyzed macrocyclization. Angew Chem Int Ed. 2024;63(3):e202316739.[DOI]
-
67. Liu Q, Teng K, Zhang Y, Lv Y, Chi YR, Jin Z. Chemodivergent parallel kinetic resolution of paracyclophanes: Enantiomer fishing with different substrates. Angew Chem. 2024;136(46):e202406386.[DOI]
-
68. Lv Y, Mou C, Liu Q, Shu L, Cai Y, Lv X, et al. Asymmetric synthesis of planar chiral carbonitriles and amines via carbene-catalyzed kinetic resolution. Org Lett. 2024;26(8):1584-1588.[DOI]
-
69. Hong B, Shi L, Li L, Zhan S, Gu Z. Paddlewheel dirhodium(II) complexes with N-heterocyclic carbene or phosphine ligand: New reactivity and selectivity. Green Synth Catal. 2022;3(2):137-149.[DOI]
-
70. Shi SQ, Cui CC, Xu LL, Zhang JP, Hao WJ, Wang J, et al. Enantioselective synthesis of saddle-shaped eight-membered lactones with inherent chirality via organocatalytic high-order annulation. Nat Commun. 2024;15(1):8474.[DOI]
-
71. Zhang XY, Zhu D, Cao RF, Huo YX, Ding TM, Chen ZM. Enantioselective synthesis of inherently chiral sulfur-containing calix[4]arenes via chiral sulfide catalyzed desymmetrizing aromatic sulfenylation. Nat Commun. 2024;15(1):9929.[DOI]
-
72. Du CB, Long YJ, Han XN, Han Y, Chen CF. Recent advances in novel chiral macrocyclic arenes. Chem Commun. 2024;60(92):13492-13506.[DOI]
-
73. Dočekal V, Lóška L, Kurčina A, Císařová I, Veselý J. Desymmetric esterification catalysed by bifunctional chiral N-heterocyclic carbenes provides access to inherently chiral calix[4]arenes. Nat Commun. 2025;16(1):4443.[DOI]
-
74. Wei L, Chen Y, Zhou Q, Wei Z, Tu T, Ren S, et al. Carbene-catalyzed intramolecular cyclization to access inherently chiral saddle-shaped lactones: Achiral bases alternate product chirality. J Am Chem Soc. 2025;147(34):30747-30756.[DOI]
-
75. Wang Y, Wu ZG, Shi F. Advances in catalytic enantioselective synthesis of chiral helicenes and helicenoids. Chem Catal. 2022;2(11):3077-3111.[DOI]
-
76. Li C, Feng J, Liu Z, Xu M, Feng J, Du D. Enantioselective de novo construction of pyran-2-one-fused helicenoids via NHC organocatalysis. Angew Chem Int Ed. 2025;64(33):e202508789.[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite