Zhong-Yong Yuan, School of Materials Science and Engineering, Nankai University, Tianjin 300350, China. E-mail: zyyuan@nankai.edu.cn
Abstract
Transition metal phosphides (TMPs) have been recognized as promising electrocatalysts for water splitting due to their high electronic conductivity, tunable structure and composition, and multifunctional active sites. Combining TMPs with other materials such as metals and compounds to form heterostructures can significantly enhance electrocatalytic performance. This review summarizes recent advances in TMP-based heterostructures for electrocatalytic water splitting. The design of electrocatalyst structures and compositions, along with their corresponding electrochemical activities, is discussed. Emphasis is placed on interfacial engineering and the synergistic effects between heterocomponents to elucidate the relationship between interfacial characteristics and catalytic performance. Finally, current challenges and future research directions for TMP-based heterostructure electrocatalysts in water splitting are proposed.
Keywords
1. Introduction
Sustainable clean energy technologies have the potential to address the pressing issues of severe environmental pollution and energy shortages faced today. Electrocatalysis, as the fundamental science underpinning these technologies, has taken a central role in their development. Electrocatalysts, being the core components of electrocatalysis, have attracted increasing attention in recent years. Currently, state-of-the-art electrocatalysts are noble metals such as platinum for the hydrogen evolution reaction (HER) and ruthenium dioxide (RuO2) and iridium dioxide for the oxygen evolution reaction (OER)[1-3]. However, their widespread industrial application is greatly limited by high cost, scarcity, and limited availability. Therefore, it is of paramount importance to develop low-cost and highly efficient electrocatalysts to enable the commercial viability of these promising technologies.
Consequently, significant efforts have been devoted to developing non-noble metal-based electrocatalysts, including transition metal compounds and metal-free materials[4-6]. Among these, transition metal phosphides (TMPs) have emerged as promising electrocatalysts due to their unique physicochemical properties, such as high electronic conductivity, tunable structure and composition, and multifunctional active sites[7,8]. Unlike many other non-noble electrocatalysts (e.g., metal oxides and sulfides), TMPs exhibit metallic or semi-metallic conductivity, which significantly enhances electron transfer rates during simultaneous HER and OER catalysis. Variations in the stoichiometric ratio of metal to phosphorus result in diverse structures and physicochemical characteristics of TMPs. Reports indicate that metal-rich TMPs possess superior electronic conductivity and chemical stability compared to phosphorus-rich TMPs[9]. TMPs have been extensively studied as efficient catalysts for the HER, where negatively charged phosphorus sites and positively charged transition metal (TM) sites act as acceptors for protons and hydrides during the reaction[10]. Additionally, the excellent OER activity of TMPs has been investigated, with several studies suggesting that the true OER active sites are gradually formed metal oxide/hydroxide species on the TMP surface[11-13].
As is well known, both HER and OER are surface-sensitive reactions that primarily occur at the solid/liquid/gas three-phase interface
Herein, this review summarizes recent advances in TMP-based heterostructure electrocatalysts for water splitting, with a focus on the Mott-Schottky effect, support effect, and synergistic effect (Figure 1). The discussion encompasses the design of electrocatalyst structures and compositions and their corresponding electrochemical performance. Particular emphasis is placed on interfacial engineering and the synergistic interactions among different components. Finally, the existing challenges and future prospects of TMP-based heterostructure electrocatalysts for water splitting are presented.
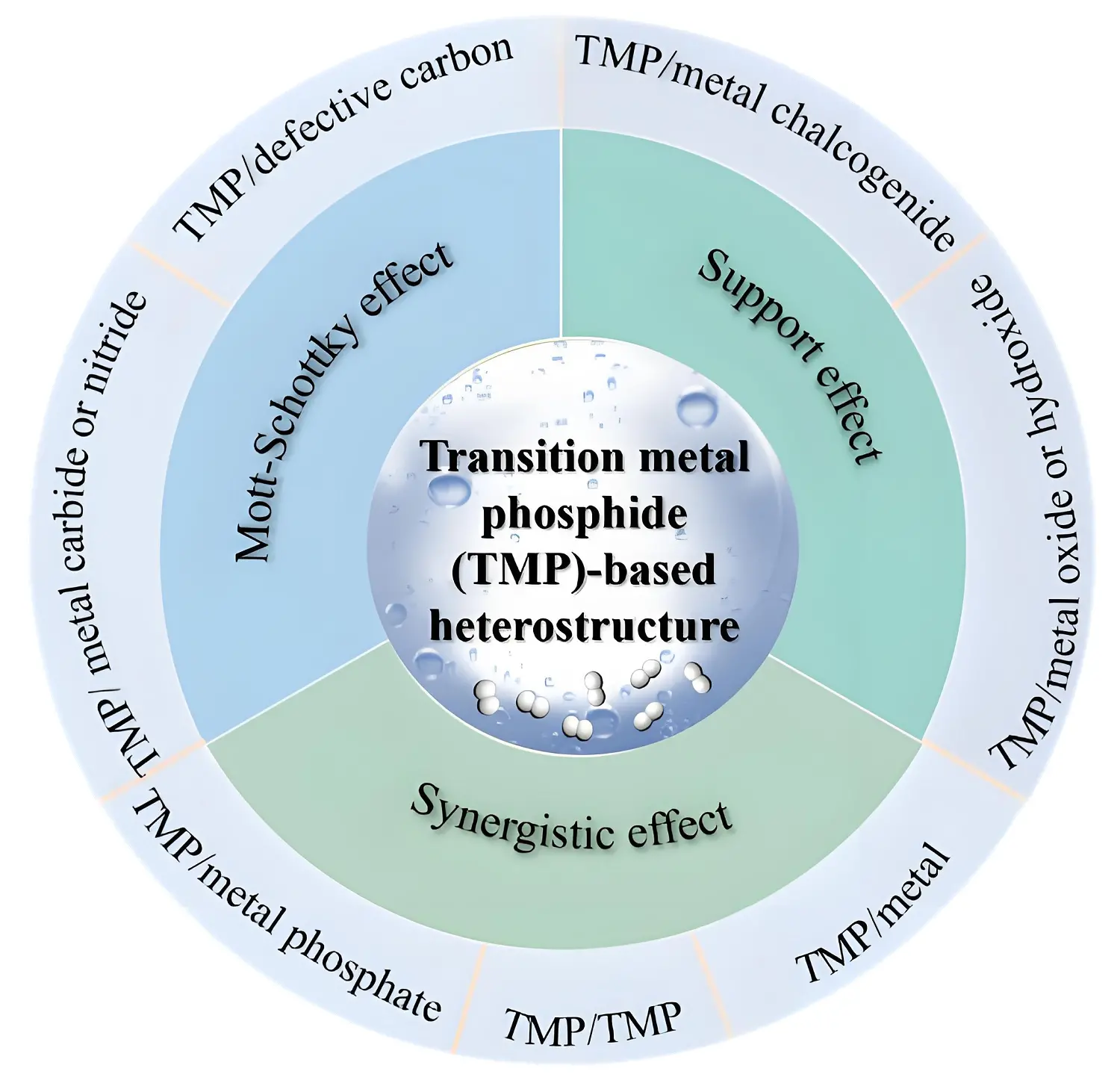
Figure 1. Illustration of TMP-based heterostructures in electrocatalytic water splitting. TMP: transition metal phosphide.
2. The Effects of TMP-Based Heterostructures
The design of TMP-based heterostructures harnesses three key effects to improve electrocatalytic performance in water splitting: the Mott-Schottky effect, the support effect, and the synergistic effect. Collectively, these effects enhance both catalytic activity and stability for the HER and OER.
2.1 Mott-Schottky effect
A Mott-Schottky heterojunction forms between a metal (or metallic compound) and a semiconductor (TMP), resulting in a space-charge region and a built-in electric field (BIEF) due to the difference in work function. This difference drives spontaneous electron transfer at the interface, inducing charge redistribution and creating an internal electric field that enhances active site accessibility and accelerates charge transfer rates[26-29]. Similarly, p-n junctions leverage band alignment to promote charge separation. Furthermore, the surface chemisorption properties toward reaction intermediates can be finely tuned, which favors accelerated reaction kinetics. This adjustment optimizes the binding energies of intermediate species and reduces energy barriers, thereby improving catalytic activity. Overall, the formation of a Mott-Schottky heterojunction allows the catalyst to integrate the intrinsic advantages of its constituent materials while generating interfacial synergy that significantly enhances catalytic performance.
2.2 Support effect
Constructing TMP/defective carbon heterostructures by combining TMPs with defective carbon materials has attracted considerable attention. Various defective carbons, such as graphene quantum dots, carbon nanotubes, graphene, and porous carbons, have been widely reported[30-33]. Strong interfacial interactions between TMPs and defective carbons facilitate the precise construction of heterostructures. Defective carbon materials typically exhibit high electrical conductivity and excellent resistance to acidic and alkaline environments. Therefore, coupling TMPs with defective carbon supports not only enhances electron transfer but also mitigates electrolyte-induced corrosion, thereby improving both the catalytic activity and stability of TMPs. The catalytic performance is strongly influenced by the properties of the carbon material. Compared to individual TMPs, incorporating defective carbon can modulate the electronic configuration and fine-tune the adsorption-desorption energetics of intermediates. Consequently, integrating TMPs with well-designed carbon matrices has become an effective strategy to achieve enhanced catalytic efficiency and long-term durability.
2.3 Synergistic effect
It is widely recognized that the rational design of multicomponent heterostructures can integrate the advantages of individual components to enhance specific properties, such as electronic conductivity and charge distribution. These heterostructures typically exhibit strong interfacial interactions, which result in improved electron transfer rates, optimized adsorption energies, and accelerated reaction kinetics[34-36]. The deliberate construction of heterojunctions thus facilitates more favorable catalytic pathways. The pronounced synergistic effect between different components at the heterointerface plays a crucial role in boosting catalytic activity. Therefore, designing abundant multilevel interfaces and multifunctional interfacial sites is essential. Computational studies have revealed that interfacial synergy enables precise electronic structure engineering and optimizes the adsorption energetics of key intermediates, thereby significantly accelerating electrocatalytic reaction rates.
3. Activity Regulation Strategies of TMP-Based Heterostructures Towards Electrocatalytic Water Splitting
Common synthesis routes for TMP-based heterostructures include solvothermal/hydrothermal methods, electrodeposition, and phosphidation, among others. The catalytic performance of TMP-based heterostructures in water splitting can be systematically optimized by strategically regulating their electronic structure, interfacial interactions, phase composition, and support architecture. These approaches work synergistically to enhance charge transfer kinetics, fine-tune the adsorption energies of reaction intermediates, and improve structural stability, thereby delivering superior electrocatalytic activity and durability.
3.1 Electronic structure optimization
Heterojunctions formed between TMPs and secondary components generate a BIEF due to differences in work function. This intrinsic field drives spontaneous electron transfer, redistributes charges at the interface, and reduces energy barriers for water dissociation and intermediate adsorption[37,38]. For example, Lin et al. constructed a Ni2P/CoP heterojunction anchored on nickel foam (Ni2P/CoP-NF) via a two-step phosphorization process, creating a robust p-n heterojunction that establishes a stable BIEF[39]. This design promotes continuous electron transfer from CoP to Ni2P, thereby optimizing the local electronic states of active catalytic centers during electrocatalysis. As a result, the catalyst demonstrates outstanding bifunctional performance, achieving low overpotentials of 238 mV for OER and 101 mV for HER at 50 mA·cm-2, a cell voltage of 1.91 V for overall water splitting at 500 mA·cm-2, and stability over 300 hours. In situ Raman spectroscopy and density functional theory (DFT) studies further confirm that the BIEF accelerates the formation of active CoOOH and Co(OH)2 intermediates, reducing energy barriers for *OH deprotonation (OER) and H2O dissociation (HER). Similarly, Yan et al. designed a NiCoP-Co/MXene Mott-Schottky heterostructure, where the work function difference (ΔΦ = 0.43 eV) between Co and NiCoP drives spontaneous electron transfer, creating an asymmetric charge distribution at the interface[40]. The incorporation of MXene not only enhances electrical conductivity but also prevents nanosheet aggregation, resulting in excellent catalytic performance: a low cell voltage of 1.52 V at 10 10 mA·cm-2 in 1 M KOH, stable operation for 50 hours, and remarkable overpotentials of 55 mV for HER and 236 mV for OER at 10 mA·cm-2, outperforming Pt/C and RuO2 benchmarks. These studies collectively highlight the critical role of BIEF in tuning adsorption energies of key intermediates and accelerating reaction kinetics for efficient water splitting.
The electronic structure of TMP-based heterostructures can also be optimized through d-band center regulation[41]. A widely adopted strategy is heteroatom doping, as demonstrated by Br-induced Co1.085P@NF, where the incorporation of bromine caused a negative shift in the Co d-band center. This shift weakened the adsorption of oxygen intermediates and reduced energy barriers, thereby enhancing catalytic efficiency[42]. Combined with ultra-hydrophilicity, this design achieved remarkable performance, with overpotentials of 90 mV (HER) and 197 mV (OER) at 50 mA cm-2, and a low cell voltage of 1.59 V for overall water splitting. Similarly, the construction of Co4S3/CoP3 heterostructures facilitated interfacial charge redistribution, inducing a downward shift in the d-band center to optimize the adsorption/desorption balance of intermediates[43]. This heterostructure exhibited outstanding OER activity, delivering overpotentials of 190 mV and 272 mV at 20 and 50 mA cm-2, respectively, surpassing both monophase counterparts and RuO2 benchmarks.
3.2 Interfacial engineering
Interfacial interactions between TMPs and other components (e.g., oxides, carbides, or carbon supports) play a critical role in determining catalytic efficiency. Introducing defects or lattice strain at these interfaces creates unsaturated coordination sites and enhances electron delocalization. For instance, compressive strain in Ni2P/Co2P heterostructures, achieved by “similar stacking” of hexagonal Co2P on Ni2P, optimized the hydrogen adsorption free energy (ΔGH* = 0.23 eV) and delivered a HER current density of
Dynamic surface reconstruction during operation is also crucial for activating catalytic phases. For instance, Wei et al. introduced phosphorus vacancies (Pv) into CoFeP via Ar plasma treatment, inducing electron redistribution, lowering the energy barrier for *H2O dissociation, and achieving an HER overpotential of 367 mV at 1 A cm-2 under industrial conditions (6 M KOH, 80 °C)[48]. In situ Raman analysis revealed that P vacancies accelerated phase reconstruction into CoFeOOH during OER, reducing the overpotential to 382 mV at 1 A cm-2. Similarly, a Ce-doped Fe2P/NiCoP hybrid pre-catalyst was designed to enhance OER kinetics for anion exchange membrane water electrolysis (AEMWE)[49]. The optimized Ce0.1-Fe2P/NiCoP exhibited an extremely low OER overpotential of 280 mV at 0.5 A cm-2 and a small Tafel slope of 55.3 mV dec-1 in 1.0 M KOH, outperforming RuO2 benchmarks. When integrated into an AEMWE electrolyzer, it delivered a cell voltage of 1.812 V at 1.0 A cm-2 and maintains stable operation for over 500 hours at 60 °C. In situ characterizations and DFT calculations revealed that Ce doping promoted dynamic surface reconstruction into Ce-FeOOH/NiCoP, optimizing the electronic structure, lowering energy barriers for intermediate formation, and enhancing charge transfer. The synergistic combination of Ce doping, bimetallic hybridization, and retained metallic NiCoP phases significantly improved both catalytic activity and long-term durability.
3.3 Phase regulation
Crystalline phases, characterized by long-range atomic order, provide excellent electrical conductivity and structural stability, enabling rapid electron transport. In contrast, amorphous phases, with their disordered atomic arrangement, expose abundant unsaturated coordination sites and defects, thereby increasing the density of active sites and modulating adsorption energy barriers for key intermediates. The synergistic integration of crystalline and amorphous phases through heterointerface engineering promotes interfacial charge redistribution, tunes electronic structures, and accelerates reaction kinetics. Thus, rational design of crystalline/amorphous heterojunctions represents a promising approach to enhance electrocatalytic performance, offering efficient, durable, and scalable solutions for hydrogen production via water electrolysis.
For example, CoP@WP2/NF core-shell nanowires (featuring a crystalline CoP core and an amorphous WP₂ shell) deliver ultralow overpotentials of 13 mV (HER, acidic) and 254 mV (OER, alkaline) at 10 mA cm-2, outperforming Pt/C and RuO₂ benchmarks[50]. The amorphous WP2 shell increases active site density and corrosion resistance, while interfacial charge coupling between CoP and WP2 reduces kinetic barriers. Similarly, CoP/FeCoPX hierarchical nanoarrays employ Fe doping and phosphorus vacancies to achieve HER and OER overpotentials of 74 mV and 237 mV in alkaline media, respectively, with a full-cell voltage of 1.53 V at 10 mA cm-2, underscoring the role of electronic engineering in optimizing intermediate adsorption[51]. The NiCoP/Co2P@NF core-shell structure achieves an ultralow HER overpotential of 26 mV at 10 mA cm-2, attributed to electron-rich Co sites at the amorphous-crystalline interface, where the amorphous NiCoP shell not only protects the crystalline Co2P core but also provides a large electrochemical surface area[52].
Amorphous components further facilitate dynamic surface reconstruction during catalysis. In reduced graphene oxide (RGO)-supported heterostructures comprising crystalline nickel phosphide and amorphous iron phosphate phases (Ni2P/FePOX/RGO), the amorphous FePOX promotes the in situ formation of active oxyhydroxides under OER conditions, enabling outstanding performance at industrial current densities (421 mV at 1000 mA cm-2)[53]. Similarly, a medium-entropy NiCoFeP@NiCoFe-layered double hydroxide (LDH) architecture, which combines crystalline phosphide nanowires with amorphous LDH nanosheets, achieves HER/OER overpotentials of 46 mV and 148 mV at 10 mA cm-2 and sustaining 1,000 mA cm-2 for 600 hours[54].
3.4 Support engineering
Defective carbon substrates, characterized by heteroatom doping, hierarchical porosity, and tailored nanostructures, play a crucial role in enhancing catalytic activity by improving electrical conductivity, exposing abundant active sites, and facilitating rapid electron/ion transport.
For example, nitrogen-doped porous carbon (NC) in NiFeP/NC hybrids, synthesized through KOH activation and ammonia annealing, provides a three-dimensional hierarchical structure that promotes lattice oxygen activation[55]. N-doping redistributes electron density, lowers the energy barrier for oxygen evolution via the lattice oxygen oxidation mechanism, and achieves an OER overpotential of 241 mV at 10 mA cm-2. The porous framework also prevents nanoparticle aggregation and improves mass transport. Similarly, nickel-nitrogen co-doped carbon nanofibers (Ni-NCNF) in NiFeP@Ni-NCNF leverage Ni-N co-doping to enhance electrical conductivity and create a superhydrophilic/superaerophobic surface, accelerating bubble detachment and electrolyte penetration[56]. Coupled with vertically aligned NiFeP nanosheet arrays, this design achieves low bifunctional overpotentials (HER: 131 mV, OER: 204 mV) and an overall cell voltage of 1.61 V, attributed to maximized active site exposure and efficient charge transfer. Additionally, S-doped carbon nanobelts (SC) in Co2P@SC modulate electron distribution via sulfur doping, strengthening proton adsorption for HER (78 mV) and optimizing intermediate binding for OER (242 mV)[57]. The interconnected nanobelt network prevents Co2P agglomeration and facilitates efficient mass and charge transport, resulting in a cell voltage of 1.57 V.
Nanostructure-dependent effects also play a pivotal role. For instance, ultrafine N-doped carbon nanotubes (U-NCNTs) in
Overall, the catalytic performance of TMP-based heterostructures in water splitting can be significantly improved through strategic regulation of electronic structures, interfacial interactions, phase compositions, and substrate engineering. Tailoring electronic configurations via heterojunction formation or heteroatom doping optimizes the d-band center, balancing adsorption/desorption energies of intermediates and reducing kinetic barriers. Interfacial engineering, through defects, lattice strain, and dynamic surface reconstruction, enhances charge transfer and promotes the exposure of metastable active phases. Synergistic integration of crystalline and amorphous phases ensures structural stability while increasing active site density to accelerate reaction kinetics. Finally, incorporating defective carbon supports improves conductivity, mitigates nanoparticle aggregation, and tunes electronic states, collectively enabling high activity and industrial-level durability.
4. Applications of TMP-Based Heterostructures in Electrocatalytic Water Splitting
TMP-based heterostructures demonstrate broad applicability in electrocatalytic water splitting, primarily enabled by precise interfacial engineering. These architectures deliver outstanding performance metrics, including ultralow overpotentials, high current densities, and long-term operational stability under industrial conditions. Such characteristics underscore their potential as scalable and cost-effective catalysts for next-generation clean energy technologies.
4.1 TMP/metal heterostructure
A heterostructured Co-Co2P catalyst embedded in a three-dimensional N, P dual-doped carbon matrix further modified with carbon nanotubes (denoted as Co-Co2P@CNT//CM) was synthesized using sodium hypophosphite (NaH2PO2)-modified Zeolitic Imidazolate Framework-67 (ZIF-67) as the precursor[59]. Work function analysis revealed a higher value for Co2P (4.80 eV) compared to Co (4.73 eV)
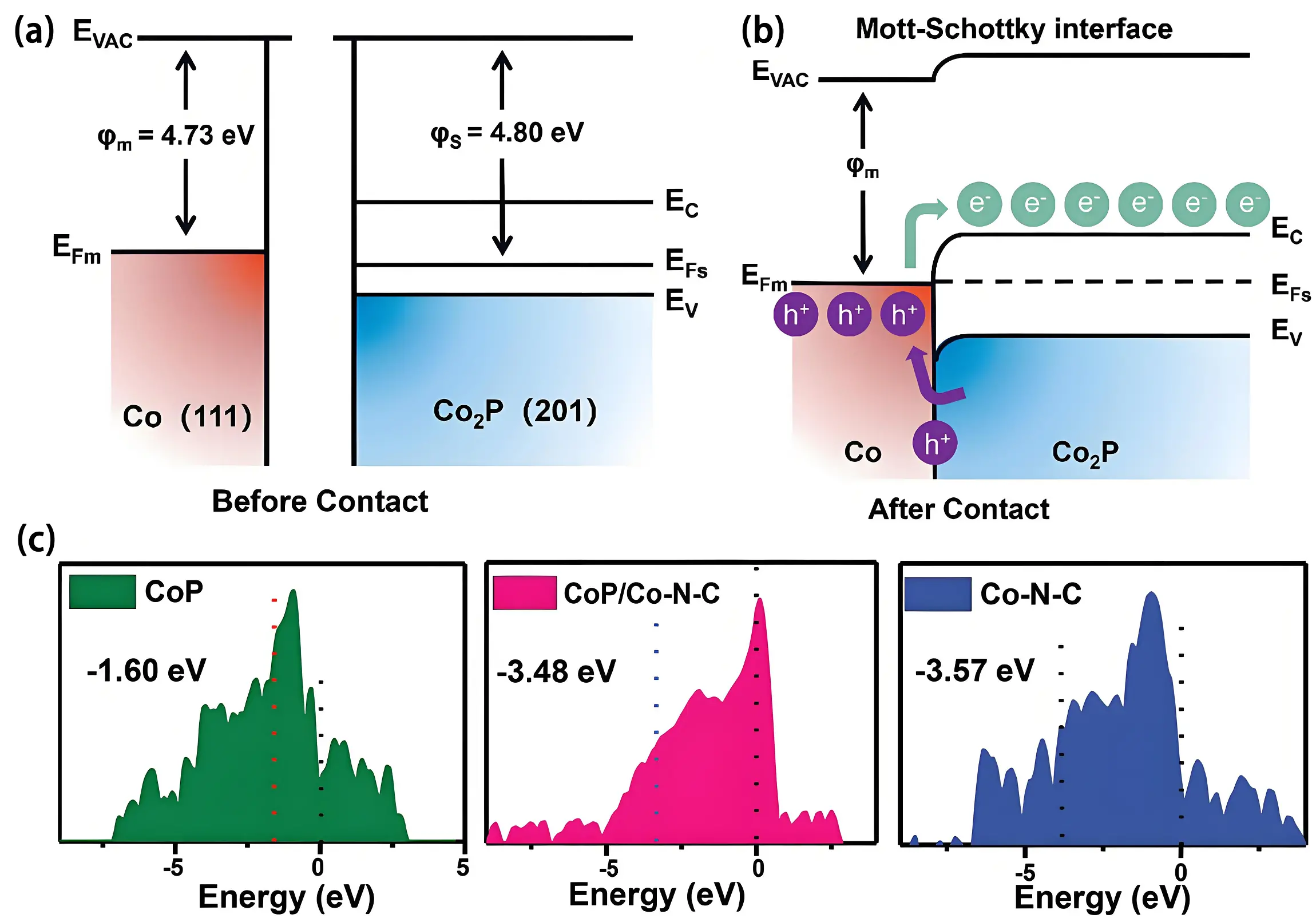
Figure 2. Energy band diagrams illustrate Co and Co2P (a) pre- and (b) post-Schottky junction formation. Republished with permission from[59]; (c) Calculated d-band density of state for CoP, CoP/Co-N-C and Co-N-C. Republished with permission from[60]. EVAC: vacuum energy; EFm: Fermi level of metal; EFs: Fermi level of semiconductors; φm: vacuum electrostatic potential of metal; φs: vacuum electrostatic potential of semiconductors; EC: conduction band; EV: valence band.
Additionally, DFT simulations revealed that Mott-Schottky heterostructures can tune the d-band center to balance oxygen intermediate adsorption and desorption[60]. A CoP/Co-N-C heterojunction was synthesized via controlled phosphorization of
4.2 TMP/defective carbon heterostructure
Incorporating heteroatoms such as nitrogen (N) and phosphorus (P) into carbon supports effectively tunes the electronic properties of electrocatalysts and generates additional active sites, thereby enhancing catalytic efficiency. For example, a three-dimensional cobalt phosphide nanoparticle embedded into N, P co-doped carbon nanotubes knitted hollow nanowall arrays on carbon textiles (CoPʘNPCNTs/CTs) was synthesized using a cobalt metal-organic framework (Co-MOF) as the precursor (Figure 3a)[64]. The catalytic synergy between the CoP phase and the NPCNT support renders the composite highly active for both HER and OER. The CoPʘNPCNTs/CTs exhibit overpotentials of 101.9 mV in 1.0 M KOH and 53.1 mV in 0.5 M H2SO4 for HER at 10 mA cm-2. When used as both the anode and cathode in a water electrolyzer, the system requires a cell voltage of 1.66 V to reach 200 mA cm-2 (Figure 3b). Combined experimental and computational studies confirm that both the NPCNTs and the nitrogen, phosphorus-coating contribute to lowering the apparent activation energy barrier for HER (Figure 3c).
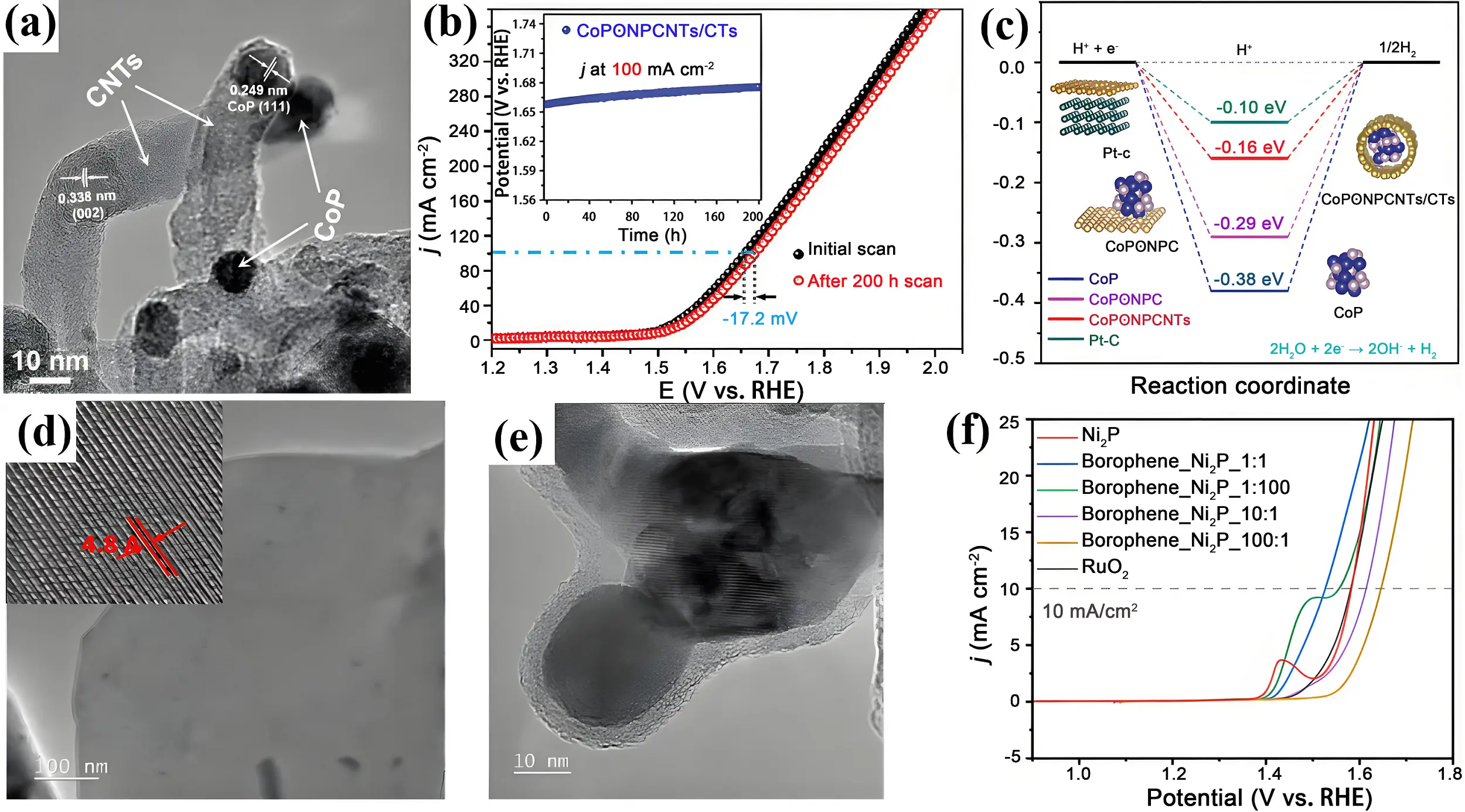
Figure 3. (a) HRTEM images of hollow CoPʘNPCNTs nanowall; (b) Polarization curves depicting the HER/OER activity of CoPʘNPCNTs/CTs initially and following a 200-hour stability test. The inset chronopotentiometry curve demonstrates catalyst stability under a fixed potential at 100 mA cm-2 for 200 h; (c) Calculated free energy diagrams illustrating H* adsorption intermediates on catalyst surfaces during the HER. Republished with permission from[64]; (d) TEM image of borophene nanosheets. The inset presents the inverse FFT pattern of the selected region of the HR-TEM image indicating the interlayer distance; (e) TEM image of borophene_Ni2P. (f) LSV plot of the OER for catalysts in 1 M KOH[65]. HR-TEM: high-resolution transmission electron microscopy; CoP: cobalt phosphide; NPCNTs: nitrogen-doped porous carbon nanotubes; HER: hydrogen evolution reaction; OER: oxygen evolution reaction; CTs: carbon textiles; FFT: Fast Fourier Transform; HR-TEM: high-resolution transmission electron microscopy; LSV: linear sweep voltammetry; CNTs: carbon nanotubes.
Defect-rich carbons have also been reported to optimize the band structure of TMPs. For instance, CoP nanoparticles confined within a defect-rich carbon shell (CoP/DCS) were synthesized via self-assembly of modified polycyclic aromatic molecules[66]. This method enables precise control over crystalline defects in the carbon matrix through strategic grafting and subsequent removal of C-N bonds from the precursor molecules. DFT calculations demonstrate that defects with unpaired electrons in the carbon shell significantly tailor the electronic band structure of encapsulated CoP nanoparticles. As a result, CoP/DCS exhibits excellent catalytic performance with overpotentials of 88 mV (HER) and 251 mV (OER) at 10 mA·cm-2. When applied as both cathode and anode in a water electrolyzer, the system delivers a cell voltage of 1.49 V at 10 mA·cm-2 and maintains good stability over 24 hours.
Beyond defective carbon supports, borophene, a monolayer boron nanostructure, has been utilized as a support (Figure 3d). Borophene features exceptional carrier mobility, mechanical robustness, and superconductivity. Nickel phosphide nanoparticles supported on borophene (borophene Ni2P, 1:1) were synthesized and employed as an OER electrocatalyst (Figure 3e)[65]. This catalyst demonstrated high activity with an overpotential of 299 mV and excellent durability for OER in 1 M KOH solution (Figure 3f). The presence of borophene prevents Ni2P nanoparticle agglomeration and enhances charge transfer, thereby boosting OER performance.
4.3 TMP/TMP heterostructure
Research indicates that CoP, characterized by abundant Co-P bonds, effectively provides additional proton-acceptor sites for the HER, while Co2P, featuring numerous Co-Co bonds, accelerates the formation of the OER intermediate OOH*[67]. Accordingly, Fe-doped
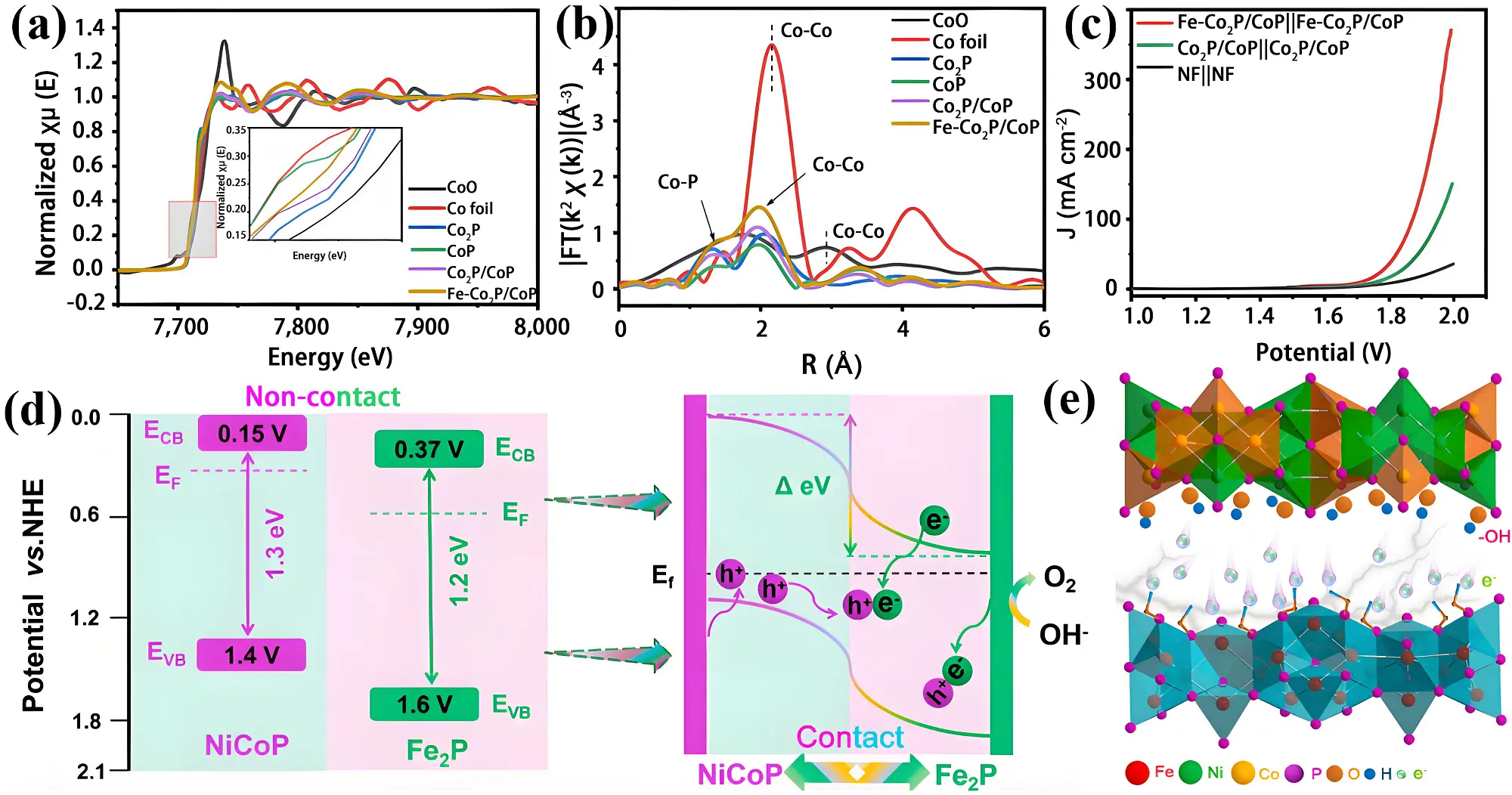
Figure 4. (a) The Co K-edge XAFS spectra; (b) k2-weighted χ(k)-function of Co-XAFS spectra of Fe-Co2P/CoP, Co2P/CoP, CoP, Co2P, CoO and Co foil; (c) LSV curves of Fe-Co2P/CoP||Fe-Co2P/CoP and
Compared with single-metal phosphides, multi-metal phosphides exhibit synergistic effects between metal species, which favorably regulate microstructure, accelerate charge transfer, and increase conductivity and active surface area[70-72]. For example, the CoP/CoMoP2 heterojunction was synthesized via a CoMo-layered double hydroxide precursor[73]. The interfacial interaction between CoP and CoMoP2 leads to charge redistribution, enhancing catalyst conductivity and modulating the adsorption of H2O and H*. The CoP/CoMoP2 exhibited good HER activity with an overpotential of 93.6 mV at 10 mA cm-2 in 1 M KOH.
Furthermore, a BIEF can be generated at the heterojunction interface. The Fe2P/NiCoP heterostructure was synthesized via a solvothermal method[69]. As illustrated in Figure 4d, upon intimate contact within the heterojunction, electrons transfer from NiCoP to Fe2P, equilibrating their Fermi levels through downward and upward shifts in NiCoP and Fe₂P, respectively. This electron redistribution establishes a strong BIEF at the interface. The resulting positive charge accumulation on the NiCoP surface facilitates the recombination of electrons, supplied by OH- ions, with holes located in the NiCoP valence band. Operando Raman analysis reveals that the BIEF promotes surface reconstruction of NiCoP, converting it into catalytically active NiCoOOH species (Figure 4e). This structural transformation modulates the binding energies of intermediates during the OER reactions, leading to enhanced catalytic performance. Consequently, the catalyst exhibits excellent OER activity, with overpotentials of 247 mV and 255 mV required to achieve 10 mA cm-2 in alkaline freshwater and simulated seawater, respectively.
Trimetallic phosphides encapsulated within N-doped carbon nanofibers (CoNiP/CoNiFeP@NCNFs) were synthesized using a CoNi coordination polymer and CoNiFe layered double hydroxide as precursors[74]. The CoNiP/CoNiFeP@NCNFs exhibited excellent catalytic performance for both OER and HER, achieving overpotentials of 260 mV and 177 mV, respectively, in 1 M KOH. When used as both the anode and cathode in a water electrolyzer, the system required a cell voltage of 1.55V to reach 10 mA cm-2. The enhanced catalytic performance originates from the synergistic interactions among CoP, FeP, and Ni2P, which facilitate interfacial electron transport. At the same time, Fe doping modulates the valence states of Co and Ni, thereby optimizing the adsorption energetics of oxygen-containing intermediates. Additionally, a simple one-pot pyrolysis strategy was employed to anchor composition-tuned Mn₂P-MnP heterojunction nanoparticles onto a P/N-doped porous carbon network[34]. This design achieved ultralow overpotentials of 63 mV (acidic) and 98 mV (alkaline) at 10 mA cm-2, with Tafel slopes of 41 mV dec-1 and 48 mV dec-1, respectively, outperforming individual Mn2P or MnP phases. DFT calculations revealed that the Mn2P-MnP interface optimized ΔGH* to 0.057 eV, close to the thermoneutral value, while the carbon matrix enhances stability and charge transfer.
4.4 TMP/ metal oxide or hydroxide heterostructure
Interfacial engineering through in situ self-reconstruction dynamically generates active metal hydroxide/oxide layers, where the core-shell structure stabilizes the interface and modulates electronic states. It has been demonstrated that some catalysts, such as metal phosphides, undergo in situ self-reconstruction during oxidation reactions, transforming into corresponding metal oxides/hydroxides, which are considered the actual active sites. Accordingly, various metal oxides including CrOx, CoO, NiO, Fe3O4, CeO2 and CoNiOx have been reported to composite with TMP to construct heterostructures[75-80]. The synergistic effect between TMPs and metal oxides or hydroxides promotes charge transfer and modifies electronic structures to increase the number of active sites. For example, phosphidated cobalt oxide with a nanoscale heterointerface (PCO-nHI) was fabricated by a sol-gel reaction combined with a hot injection method, followed by phosphidation[81]. DFT calculations demonstrated that CoO promotes water dissociation while CoP facilitates OH* removal. The presence of CoP helps maintain the stability of CoO and prevents its reduction to metallic cobalt. Consequently, PCO nHI exhibited high alkaline HER and OER activity with overpotentials of 168 mV and 396.4 mV at
Multi-component metal oxide TMP nanointerfaces have also been reported to outperform single-metal systems. Crystalline Ni2P clusters anchored on amorphous NiMoOx supported by nickel foam (Ni2P-NiMoOx/NF) were fabricated via sequential hydrothermal growth and phosphorization[82]. When deployed in water electrolysis, the system required 1.66 V to reach 100 mA cm-2 and demonstrated 100-hour durability under industrial operation (65 °C, 30% KOH) (Figure 5a). Both DFT calculations and characterizations indicated that interfacial coupling between Ni2P and NiMoOX downshifts d-band centers toward the Fermi level, facilitating water dissociation and enhancing overall electrocatalytic performance. (Figure 5b and Figure 5c).
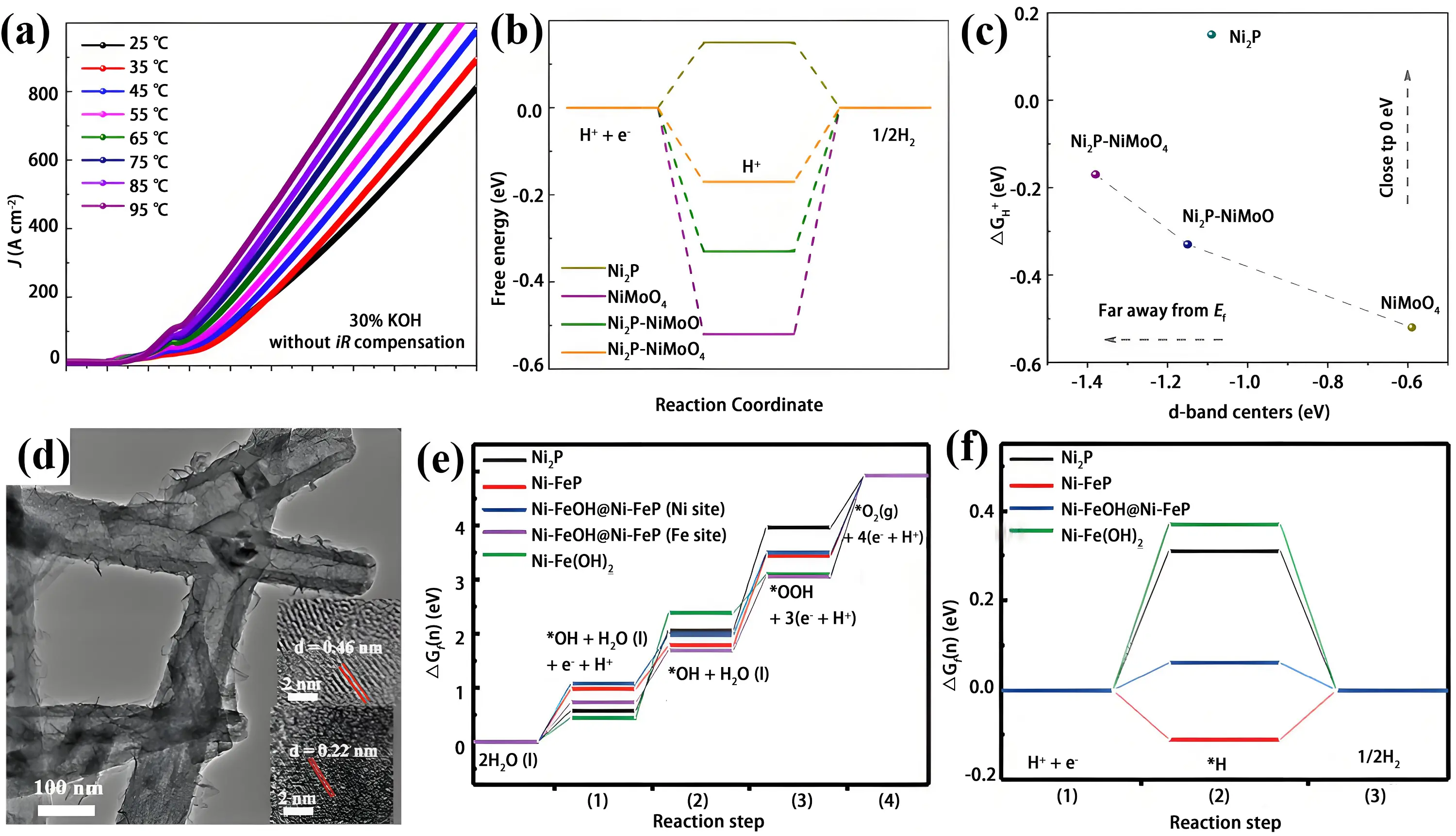
Figure 5. (a) LSV polarization curves of the Ni2P-NiMoOx/NF-catalyzed electrolyzer at various temperatures; (b) ΔGH* diagram of Ni2P, NiMoO4, Ni2P-NiMoP, and Ni2P-NiMoO4; (c) The tailoring relationship between ΔGH* and the d-band centers of εd. Republished with permission from[82]; (d) TEM image of Ni-FeOH@Ni-FeP powders with the inset of HR-TEM; The corresponding free energy diagrams for OER (e) and HER (f) on Ni2P, Ni-FeP, NiFe(OH)2, Ni-FeOH@Ni-FeP (both Ni site and Fe site) at U = 0 V. Republished with permission from[83]. LSV: linear sweep voltammetry; HR-TEM: high-resolution transmission electron microscopy; OER: oxygen evolution reaction; HER: hydrogen evolution reaction.
The heterointerface between nickel phosphide and nickel hydroxide supported on nickel foam (Ni(OH)2/Ni2P/NF) was constructed via an electrodeposition phosphorization electrodeposition method[84]. Introduction of Ni(OH)2 triggers interfacial electron density rearrangement across the Ni₂P/Ni(OH)₂ heterojunctions, suppressing charge-transfer resistance and optimizing adsorption energetics of key intermediates. The synergistic effect between Ni(OH)2 and Ni2P contributes to bifunctional catalytic activity toward both hydrazine oxidation reaction and HER, achieving potentials of -14 mV and 72 mV at 10 mA cm-2, respectively. When used as both electrodes in overall hydrazine splitting, cell voltages of 0.357 V and 0.513 V were required to deliver 100 and 200 mA cm-2 in 1 M KOH with 0.5 M hydrazine, respectively. Furthermore, the construction of core shell heterojunction structures helps maintain structural stability. As shown in Figure 5d, Ni-FeOH@Ni-FeP needle arrays with a core shell heterojunction were synthesized through in situ hydroxide growth on the Ni-FeP surface[83]. The catalyst exhibited superior intrinsic conductivity and optimized d-band center. During the OER process, the O2 release step was identified as the rate-determining step on the Fe site of Ni-FeOH@Ni-FeP, with an energy barrier of 1.86 eV (Figure 5e). The catalyst possessed the lowest Gibbs free energy for hydrogen adsorption (ΔG*H), yielding optimal catalytic activity (Figure 5f). The Ni-FeOH layer improves the structural stability of the Ni-FeP core and cooperatively interacts with Ni-FeP, modulating their electronic structures and surface microenvironments. Consequently, the Ni-FeOH@Ni-FeP-based alkaline water electrolyzer achieves a cell voltage of 2.14 V to reach 1 A cm-2 and demonstrates long-term stability over 240 hours.
4.5 TMP/ metal chalcogenide heterostructure
Metal chalcogenides, including metal tellurides, sulfides, and selenides, have shown significant potential in electrocatalysis owing to their tunable physical, chemical, and electronic properties[85-87]. Constructing TMP/metal chalcogenide heterostructures enables modulation of charge distribution, optimization of electronic structures, and acceleration of reaction kinetics[88-92].
The synergistic interaction between Ni2P and NiS2 can effectively enhance electrocatalytic performance. A nickel phosphide/sulfide (Ni2P/NiS2) microsphere electrocatalyst was synthesized via a bubble-template electrodeposition process followed by phosphorization and sulfuration (Figure 6a)[93]. NiS2 modification induces interfacial charge redistribution and strengthens Ni-P bond covalency at heterojunctions, facilitating optimized adsorption of H* intermediates and water molecules. As a result, the catalyst exhibited high activity toward both OER and HER, requiring overpotentials of 320 mV and 169 mV at 100 mA cm-2, respectively, in 1 M KOH. In alkaline seawater, the Ni2P/NiS2 electrocatalyst achieved overpotentials of 344 mV and 188 mV for OER and HER, respectively, at 100 mA cm-2 (Figure 6b).Furthermore, the formation of polyanionic species derived from NiS2 generates a protective surface film that inhibits chloride ion (Cl-) penetration (Figure 6c). This barrier improves corrosion resistance, suppresses corrosive processes, and ensures long-term durability for alkaline seawater electrolysis.
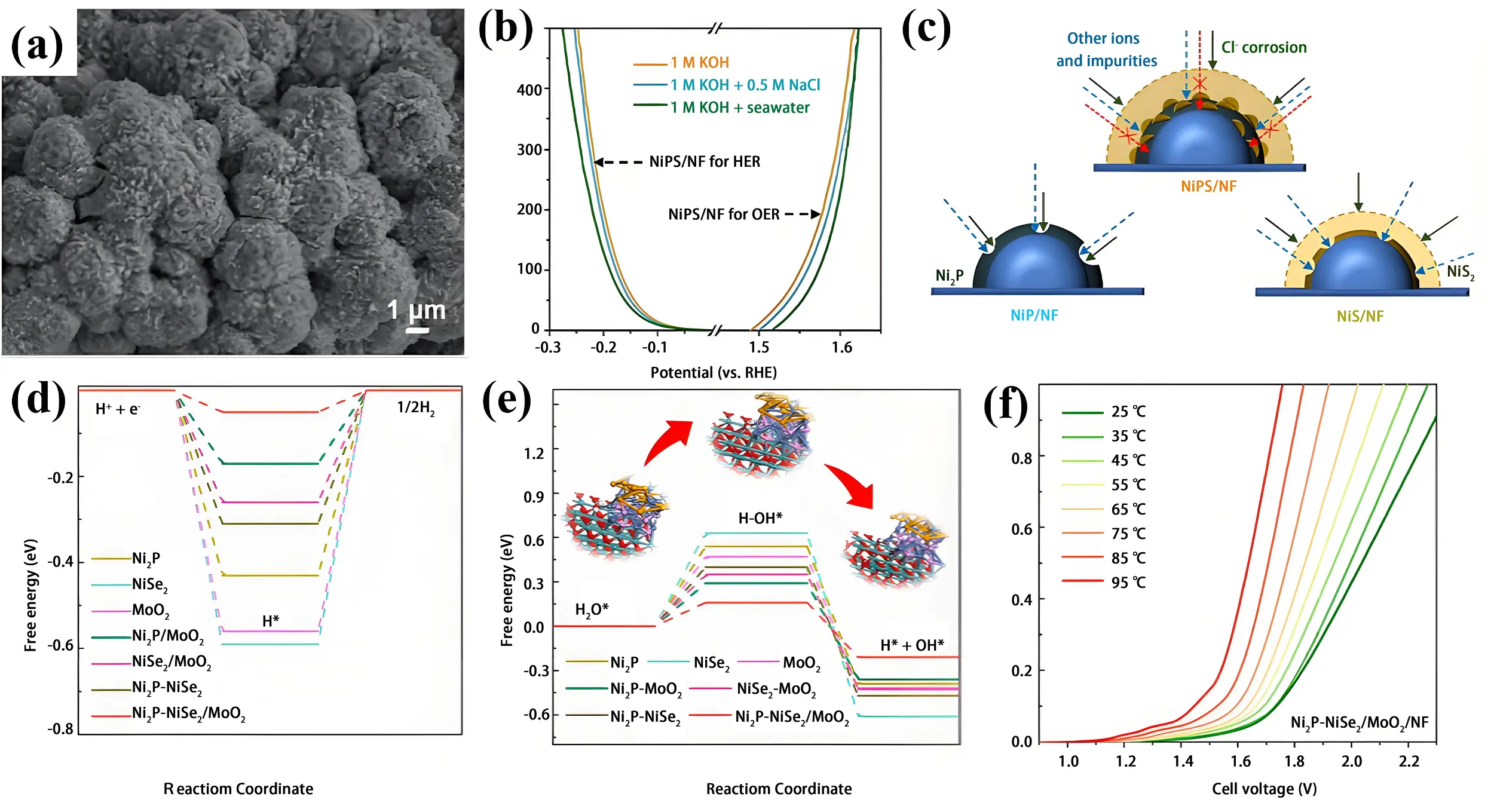
Figure 6. (a) SEM image of Ni2P/NiS2; (b) HER/OER polarization curves of Ni2P/NiS2 in 1.0 M KOH, 1.0 M KOH + 0.5 M NaCl and 1.0 M KOH + natural seawater; (c) Stability enhancement schematic for Ni2P/NiS2 anodes in seawater splitting. Republished with permission from[93]; (d) ΔGH* diagram and (e) reaction energy diagram for H2O dissociation; (f) Temperature-dependent water splitting polarization curves of Ni2P-NiSe2/MoOx/NF in 30% KOH electrolyte. Republished with permission from[94].
In addition, multi-component synergistic effects can further enhance electrocatalytic performance through strong coupling interactions among multiple active phases. For instance, Ni2P-NiSe2 were decorated on amorphous MoOx nanorods supported on nickel foam (Ni2P-NiSe2/MoOx/NF)[94]. The interfacial coupling between Ni2P, NiSe2 and MoOx optimizes charge distribution and electronic structure, accelerating the initial steps of alkaline HER (Figure 6d and Figure 6e). The resulting catalyst displayed outstanding performance, requiring overpotentials of only 241 mV for OER and 23 mV for HER at 10 mA cm-2. When applied in a water electrolyzer, the system required a cell voltage of 1.63 V at 50 mA cm-2 and maintained stability for 1,000 hours at 20 mA cm-2 in 1 M KOH. Under industrial conditions (30% KOH at 65 °C), the electrolyzer achieved 1,000 mA cm-2 at 2.02 V with remarkable durability of 200 hours at 200 mA cm-2 (Figure 6f).
4.6 TMP/ metal phosphate heterostructure
Metal phosphates, a class of multifunctional materials, exhibit tunable acidity, excellent thermal stability, and high conductivity. They have been widely reported as efficient OER electrocatalysts in both neutral and alkaline media. Structural distortions within phosphate groups can stabilize the high oxidation states of metals, thereby promoting water adsorption and oxidation on the catalyst surface[95-97]. Constructing TMP/metal phosphate heterostructures increases the number of active sites and accelerates charge transfer, leading to strong synergistic effects that enhance overall electrocatalytic performance[98-102].
Crystalline materials typically provide superior charge transport efficiency and structural stability, whereas amorphous phases offer abundant structural defects and unsaturated coordination sites[103]. Based on these complementary characteristics, crystalline/amorphous heterophase engineering of TMP/metal phosphate systems emerges as an effective strategy to improve catalytic activity. For example, crystalline Ni5P4/amorphous CePO4 core/shell heterostructure arrays were fabricated on Ni foam via in situ deposition followed by low-temperature phosphating (Figure 7a)[104]. Both experimental data and theoretical calculations revealed that amorphous CePO4 surface modification enhances the electrochemically accessible surface area and facilitates charge transfer. Moreover, CePO4 effectively modulates the d-band center of Ni5P4, optimizing intermediate adsorption and accelerating water dissociation kinetics (Figure 7b and Figure 7c). Consequently, the heterostructure delivered low overpotentials of 191 mV (OER) and 94 mV (HER) at 10 mA cm-2 in 1 M KOH. When assembled into a water electrolyzer, it achieved 10 mA cm-2 at a cell voltage of
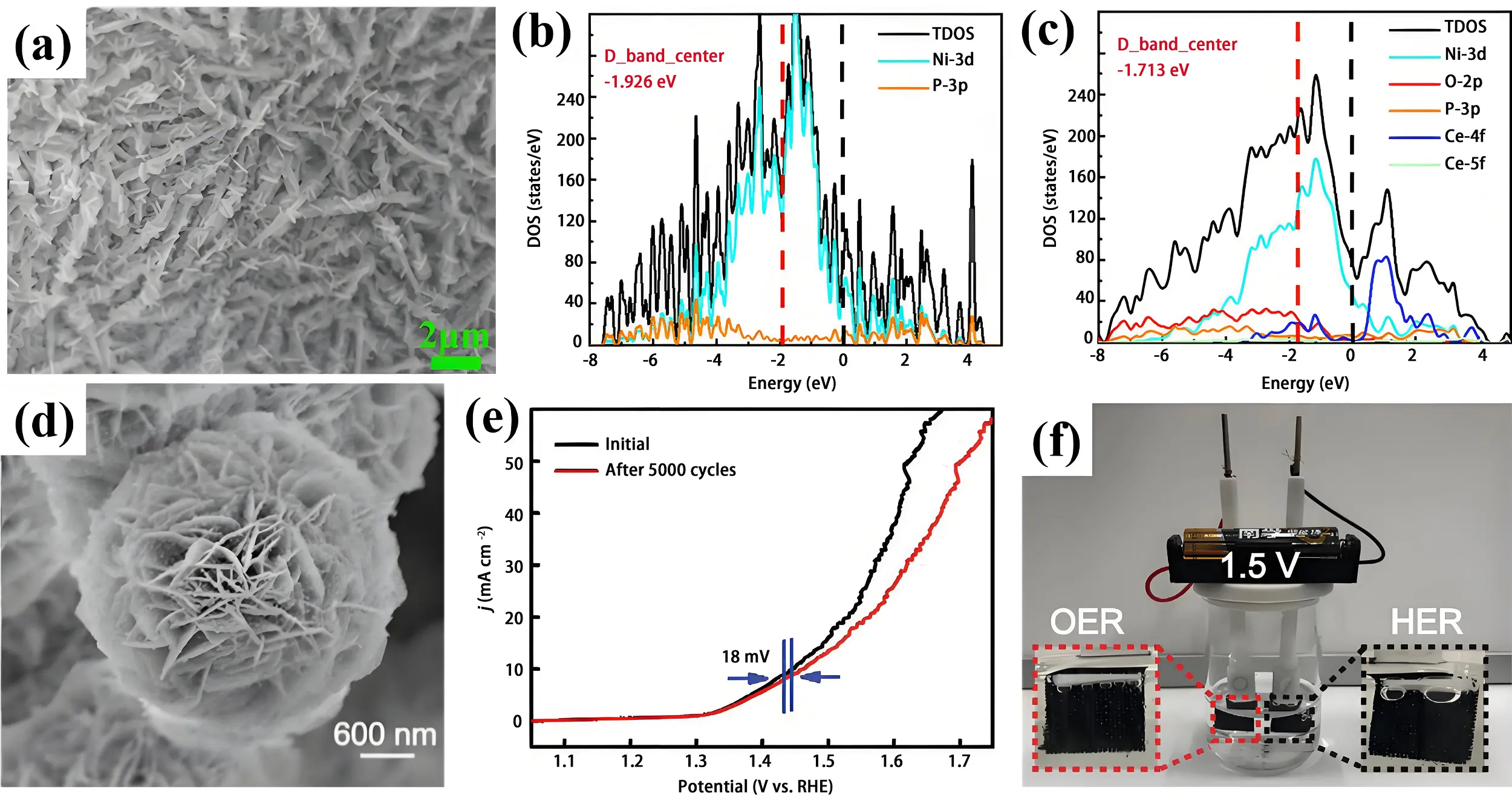
Figure 7. (a) SEM image of Ni5P4/CePO4 nanosheets heterostructure array; Electronic density of state profiles for (b) crystalline Ni5P4 and (c) crystalline Ni5P4/amorphous
Similarly, Ni2P nanocrystals immobilized on amorphous vanadium phosphate (V-Pi) nanosheets supported on carbon cloth (Ni2P/V-Pi/CC) were synthesized via oxidation and phosphorization of NiV-LDH/CC (Figure 7d)[105]. The numerous crystalline/amorphous interfaces and strong interfacial electronic interactions between Ni2P and V-Pi create abundant active sites, optimizing adsorption-desorption of reaction intermediates. Furthermore, the amorphous V-Pi phase provides excellent resistance to both acidic and alkaline environments, thereby improving long-term durability. As a result, the Ni2P/V-Pi/CC exhibited outstanding bifunctional activity, requiring overpotentials of 80.8 mV (HER) and 263 mV (OER) at 10 mA cm-2 in 1 M KOH. A water electrolyzer assembled with
4.7 TMP/ metal nitride or carbide heterostructure
Metal nitrides and carbides are interstitial compounds in which nitrogen or carbon atoms occupy interstitial positions within metallic lattices[106-108]. These materials exhibit significant potential for electrocatalytic water splitting due to their unique physicochemical properties, including noble metal-like electronic structures, high electrical conductivity, and exceptional mechanical strength[109-112]. Constructing heterostructures between TMPs and metal nitrides/carbides offers a promising approach to synergistically optimize hydrogen adsorption and water dissociation kinetics[113-115].
A Co4N/Co2P Mott–Schottky heterojunction electrocatalyst was synthesized through a hydrothermal method followed by
Another example involves an Fe2P/Co2N hybrid catalyst, in which Fe2P nanoparticles are in situ integrated onto a Co2N scaffold[117]. Theoretical calculations revealed that the strong interfacial interaction between Fe2P and Co2N optimizes the H* binding energy at Fe sites (Figure 8a). Experimentally, the heterostructure demonstrated bifunctional activity with overpotentials of 283 mV (OER) and
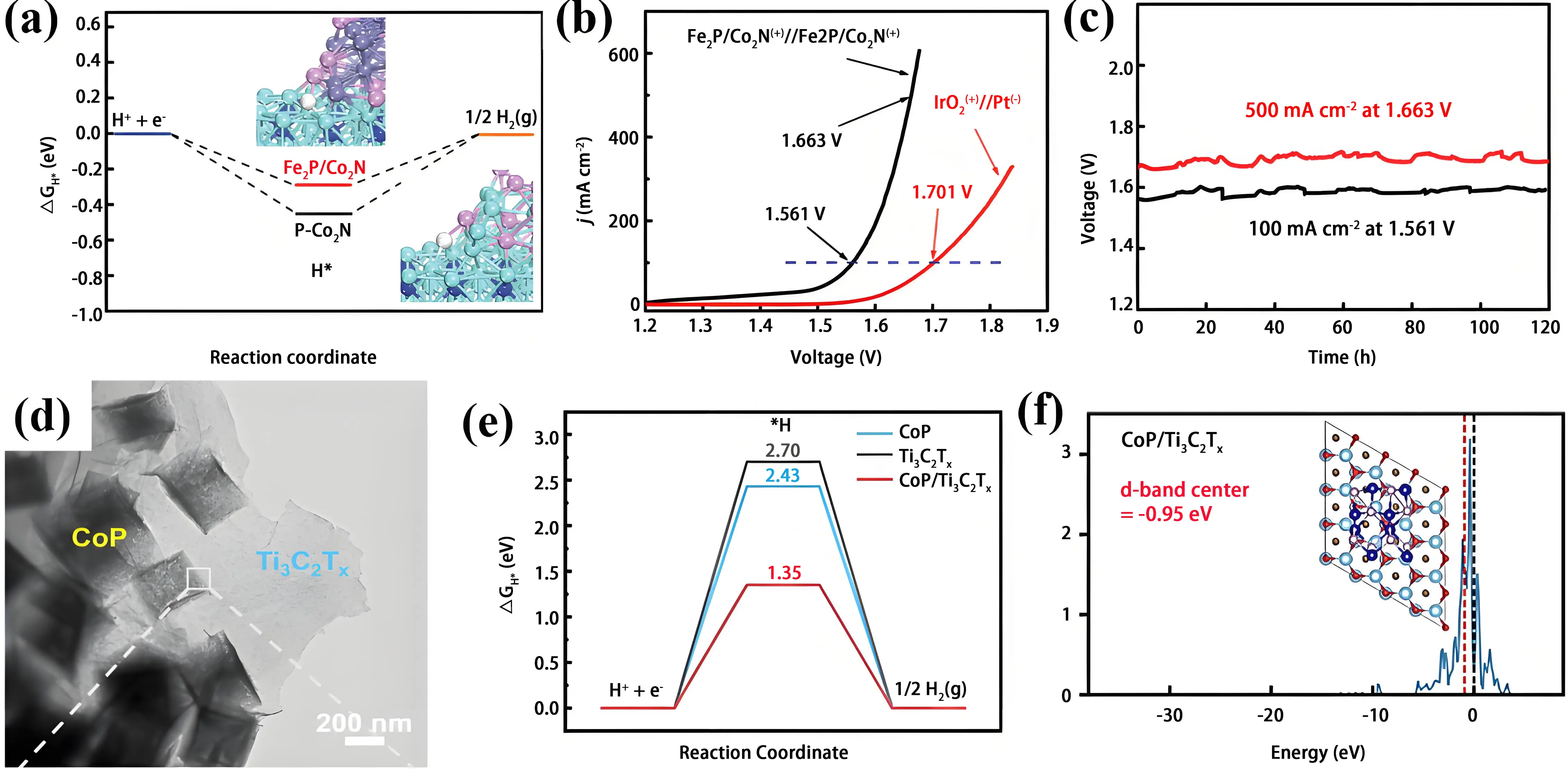
Figure 8. (a) Calculated HER Gibbs free energy landscapes at Fe₂P/Co₂N and CoP/Co₂N interfaces with optimized interfacial H* structures; (b) The overall water electrolysis polarization curves of the Fe2P//Co2N(+/-) and IrO2(+)//Pt(-) systems in 1 M KOH; (c) Long-term durability at 100/500 mA cm-2 over 120-hour continuous operation. Republished with permission from[117]; (d) TEM images of CoP/Ti3C2Tx; (e) HER free energy diagrams of Ti3C2Tx, CoP, and CoP/Ti3C2Tx; (f) Calculated d-band center of CoP/Ti3C2Tx. Republished with permission from[118]. Co: cyan; Fe: purple; N: blue; P: pink; H: white; HER: hydrogen evolution reaction; TEM: transmission electron microscopy.
To further enhance performance, multilevel interfaces and multifunctional interfacial sites have been engineered. For instance, a
Interfaces between TMPs and metal carbides also introduce abundant accessible active sites and promote efficient charge redistribution[120-122]. For example, the tungsten carbide (W2C) and tungsten phosphide (WP) embedded in N-decorated carbon
Notably, the emerging family of 2D layered MXenes, composed of transition metal nitrides, carbides, and carbonitrides, has attracted significant attention owing to their unique features, including large surface area, high electrical conductivity, and strong interfacial interactions with composite materials[124-127]. MXenes can integrate with TMPs to provide additional active sites and mitigate the agglomeration of transition metal nanoparticles, owing to their surface functional groups (-O, -OH and -F)[128].
As illustrated in Figure 8d, a CoP/Ti3C2Tx MXene heterostructure was synthesized via a coprecipitation-phosphidation method[118]. This catalyst exhibited excellent bifunctional activity, requiring overpotentials of 103 mV for HER and 312 mV for OER at 10 mA cm-2 in 1 M KOH, with stable performance maintained for 24 h. Theoretical studies revealed that the CoP/Ti3C2Tx interface enhances water adsorption on CoP, modulates the d-band center toward the Fermi level, and increases the total density of states, thereby improving conductivity and HER activity (Figure 8e and Figure 8f).
Due to the negatively charged MXene surface, electron transfer occurs from MXene to TMP at the interface, resulting in the formation of a BIEF. This BIEF modulates the electronic structure and optimizes the density of active sites, thereby improving interfacial charge transfer, accelerating electron migration, and enhancing catalytic performance[40,129,130]. Furthermore, the interfacial electronic structure and d-band center of TMP/MXene heterostructures can be further tuned through heteroatom doping and vacancy engineering in TMPs[131,132].
5. Conclusions
TMP-based heterostructures have emerged as highly promising electrocatalysts for water splitting, offering a cost-effective and efficient alternative to noble metal catalysts. This review provides a comprehensive overview of recent advancements in TMP-based heterostructures and their applications in electrocatalytic water splitting.
The Mott–Schottky effect, resulting from differences in work function between TMPs and metals or metallic compounds, drives spontaneous interfacial electron transfer. This process creates a charge redistribution and establishes a BIEF, which optimizes intermediate binding energies, lowers energy barriers, and enhances catalytic performance. The support effect highlights the critical role of conductive carbon-based materials (e.g., graphene, carbon nanotubes) in improving electronic conductivity, preventing corrosion, and tuning the electronic structure of TMPs. Meanwhile, the synergistic effect underscores interfacial coupling between TMPs and secondary components (e.g., metal oxides, chalcogenides), which enhances active site exposure, adjusts d-band centers, and facilitates reaction pathways.
Despite these significant advances, several challenges remain to be addressed before TMP-based heterostructures can achieve large-scale practical applications:
(1) Interfacial engineering as a design principle. Interfacial modulation including charge redistribution, electronic coupling, and dynamic surface reconstruction is the cornerstone for unlocking synergistic effects that integrate structural stability with catalytic activity. Precise control over interfacial structure and composition is essential for maximizing performance. However, the atomic-scale structure of interfaces and their quantitative relationship to catalytic activity remain unclear. Since multiple effects may coexist in TMP based heterostructures, isolating their individual contributions is highly challenging. Advanced in situ and operando characterization techniques (e.g., Raman spectroscopy, X-ray absorption spectroscopy(XAS)) are indispensable for decoupling these effects and establishing reliable structure-activity correlations.
(2) Long-term stability under harsh conditions. TMP based heterostructures, particularly those containing dual-metal active sites, often suffer from corrosion or phase transformations during extended operation, compromising durability. Strategies such as surface functionalization, encapsulation, and protective coatings are urgently needed to enhance stability under practical electrolysis conditions.
(3) Scalable and cost-effective synthesis. Industrial deployment demands synthesis routes that are simple, reproducible, and economically viable. However, many current methods involve complex, multi-step processes with limited scalability and low yield. Developing high-throughput, scalable synthesis strategies remains a priority for practical applications.
(4) Mechanistic understanding and computational guidance. A deeper mechanistic understanding is critical for rational catalyst design. Combining in situ experimental techniques with computational modeling will enable accurate predictions of catalytic activity and selectivity based on electronic structure and interfacial properties, thereby guiding the development of next-generation electrocatalysts.
In summary, continued research into TMP based heterostructure electrocatalysts is expected to deliver breakthroughs in electrocatalytic water splitting and other gas involving reactions, ultimately accelerating the adoption of sustainable, clean energy technologies.
Authors contribution
Zhao H: Data analysis, investigation, resources, validation, writing-original draft, writing-review & editing.
Yuan ZY: Conceptualization, project administration, supervision, validation, writing-review & editing.
All authors have given approval to the final version of the manuscript.
Conflicts of interest
Zhong-Yong Yuan is the Editor-in-Chief of Smart Materials and Devices. The other author declares no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Funding information
This work was supported by Shandong Provincial Natural Science Foundation (Grant No. ZR2023MB090).
Copyright
© The Author(s) 2025.
References
-
1. Shi Q, Zhu C, Du D, Lin Y. Robust noble metal-based electrocatalysts for oxygen evolution reaction. Chem Soc Rev. 2019;48(12):3181-3192.
[DOI] -
2. Liu Y, Wang Q, Zhang J, Ding J, Cheng Y, Wang T, et al. Recent advances in carbon-supported noble-metal electrocatalysts for hydrogen evolution reaction: Syntheses, structures, and properties. Adv Energy Mater. 2022;12(28):2200928.
[DOI] -
3. Li , Y , Sun Y, Qin Y, Zhang W, Wang L, Luo M, et al. Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials. Adv Energy Mater. 2020;10(11):1903120.
[DOI] -
4. Wu ZP, Lu XF, Zang SQ, Lou XW. Non-noble-metal-based electrocatalysts toward the oxygen evolution reaction. Adv Funct Mater. 2020;30(15):1910274.
[DOI] -
5. Bhoyate SD, Kim J, de Souza FM, Lin J, Lee E, Kumar A, et al. Science and engineering for non-noble-metal-based electrocatalysts to boost their ORR performance: A critical review. Coord Chem Rev. 2023;474:214854.
[DOI] -
6. Lv XW, Tian WW, Yuan ZY. Recent advances in high-efficiency electrocatalytic water splitting systems. Electrochem Energy Rev. 2023;6:23.
[DOI] -
7. Xiao P, Chen W, Wang X. A review of phosphide-based materials for electrocatalytic hydrogen evolution. Adv Energy Mater. 2015;5(24):1500985.
[DOI] -
8. Li X, Xing W, Hu T, Luo K, Wang J, Tang W. Recent advances in transition-metal phosphide electrocatalysts: Synthetic approach, improvement strategies and environmental applications. Coord Chem Rev. 2022;473:214811.
[DOI] -
9. Callejas JF, Read CG, Roske CW, Lewis NS, Schaak RE. Synthesis, characterization, and properties of metal phosphide catalysts for the hydrogen-evolution reaction. Chem Mater. 2016;28(17):6017-6044.
[DOI] -
10. Liu P, Rodriguez JA. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P(001) surface: The importance of ensemble effect. J Am Chem Soc. 2005;127:14871-14878.
[DOI] -
11. Liu KL, Wang FM, He P, Shifa TA, Wang Z, Cheng Z, et al. The role of active oxide species for electrochemical water oxidation on the surface of 3d-metal phosphides. Adv Energy Mater. 2018;8(15):1703290.
[DOI] -
12. Ryu J, Jung N, Jang JH, Kim HJ, Yoo SJ. In situ transformation of hydrogen-evolving CoP nanoparticles: Toward efficient oxygen evolution catalysts bearing dispersed morphologies with Co-oxo/hydroxo molecular units. ACS Catal. 2015;5:4066-4074.
[DOI] -
13. Chang J, Xiao Y, Xiao M, Ge J, Liu C, Xing W. Surface oxidized cobalt-phosphide nanorods as an advanced oxygen evolution catalyst in alkaline solution. ACS Catal. 2015;5(11):6874-6878.
[DOI] -
14. Zhao H, Yuan ZY. Surface/interface engineering of high-efficiency noble metal-free electrocatalysts for energy-related electrochemical reactions. J Energy Chem. 2021;54:89-104.
[DOI] -
15. Zhao H, Ren JT, Yuan ZY. Microenvironment engineering of gas-involving energy electrocatalysis and device applications. Coord Chem Rev. 2024;514:215901.
[DOI] -
16. Weng CC, Lv XW, Ren JT, Ma TY, Yuan ZY. Engineering gas-solid-liquid triple-phase interfaces for electrochemical energy conversion reactions. Electrochem Energy Rev. 2022;5:19.
[DOI] -
17. Wang CY, Gao MQ, Zhao CC, Zhao LM, Zhao H. Metal phosphonate-derived cobalt/nickel phosphide@N-doped carbon hybrids as efficient bifunctional oxygen electrodes for Zn-air batteries. Front Chem Sci Eng. 2022;16:1367-1376.
[DOI] -
18. Li H, Xu SM, Yan H, Yang L, Xu SL. Cobalt phosphide composite encapsulated within N,P-doped carbon nanotubes for synergistic oxygen evolution. Small. 2018;14(19):1800367.
[DOI] -
19. Yan F, Yan L, Wei X, Han Y, Huang H, Xu S, et al. Structure-design and synthesis of nickel-cobalt oxide/sulfide/phosphide composite nanowire arrays for efficient overall water splitting. Int J Hydro Energy. 2022;47(19):10616-10627.
[DOI] -
20. Zhou H, Zheng M, Pang H. Synthesis of hollow amorphous cobalt phosphide-cobalt oxide composite with interconnected pores for oxygen evolution reaction. Chem Eng J. 2021;416:127884.
[DOI] -
21. Xu J, Xiong D, Amorim I, Liu L. Template-free synthesis of hollow iron phosphide-phosphate composite nanotubes for use as active and stable oxygen evolution electrocatalysts. ACS Appl Nano Mater. 2018;1(2):617-624.
[DOI] -
22. Zhang H, Maijenburg AW, Li X, Schweizer SL, Wehrsphohn RB. Bifunctional heterostructured transition metal phosphides for efficient electrochemical water splitting. Adv Funct Mater. 2020;30(34):2003261.
[DOI] -
23. Li Y, Dong Z, Jiao L. Multifunctional transition metal-based phosphides in energy-related electrocatalysis. Adv Energy Mater. 2020;10:1902104.
[DOI] -
24. Liu D, Xu G, Yang H, Wang H, Xia BY. Rational design of transition metal phosphide-based electrocatalysts for hydrogen evolution. Adv Funct Mater. 2023;33:2208358.
[DOI] -
25. Zhang HM, Wang JJ, Meng Y, Sun J. Recent advances in amorphous metal phosphide electrocatalysts for hydrogen evolution reaction. Int J Hydro Energy. 2022;47:36084-36097.
[DOI] -
26. Xu D, Zhang SN, Chen JS, Li XH. Design of the synergistic rectifying interfaces in Mott-Schottky catalysts. Chem Rev. 2023;123:1-30.
[DOI] -
27. Krishnamachari M, Lenus S, Pradeeswari K, Arun pandian R, Kumar M, Chang JH, et al. Review of Mott-Schottky-based nanoscale catalysts for electrochemical water splitting. ACS Appl Nano Mater. 2023;6:16106-16139.
[DOI] -
28. Yang H, Wang B, Kou S, Lu G, Liu Z. Mott-Schottky heterojunction of Co/Co2P with built-in electric fields for bifunctional oxygen electrocatalysis and zinc-air battery. Chem Eng J. 2021;425:131589.
[DOI] -
29. Xue ZH, Su H, Yu QY, Zhang B, Wang HH, Li XH, et al. Janus Co/CoP Nanoparticles as efficient Mott-Schottky electrocatalysts for overall water splitting in wide pH range. Adv Energy Mater. 2017;7:1602355.
[DOI] -
30. Tian J, Chen J, Liu J, Tian Q, Chen P. Graphene quantum dot engineered nickel-cobalt phosphide as highly efficient bifunctional catalyst for overall water splitting. Nano Energy. 2018;48:284-291.
[DOI] -
31. Chen M, Liu Y, Fan J, Liu B, Shi N, Lin Y, et al. Phase-controlled synthesis of nickel-iron nitride nanocrystals armored with amorphous N-doped carbon nanoparticles nanocubes for enhanced overall water splitting. Small. 2022;18:2203042.
[DOI] -
32. Yang J, Guo D, Zhao S, Lin Y, Yang R, Xu D, et al. Cobalt phosphides nanocrystals encapsulated by P-doped carbon and married with P-doped graphene for overall water splitting. Small. 2019;15:1804546.
[DOI] -
33. Chen B, Kim D, Zhang Z, Lee M, Yong K. MOF-derived NiCoZnP nanoclusters anchored on hierarchical N-doped carbon nanosheets array as bifunctional electrocatalysts for overall water splitting. Chem Eng J. 2021;422:130533.
[DOI] -
34. Wu W, Huang Y, Wang X, Shen PK, Zhu J. Composition-optimized manganese phosphide nanoparticles anchored on porous carbon network for efficiently electrocatalytic hydrogen evolution. Chem Eng J. 2023;469:143879.
[DOI] -
35. Cao X, Tian J, Tan Y, Zhu Y, Hu J, Wang Y, et al. Interfacial electron potential well facilitates the design of cobalt phosphide heterojunctions for hydrogen evolution. Small. 2023;20(19):2306113.
[DOI] -
36. Zhang H, Liu W, Li Z, Qiao L, Chi K, Guo X, et al. Constructing CoP/Ni2P heterostructure confined Ru sub-nanoclusters for enhanced water splitting in wide pH conditions. Adv Sci. 2024;11:2401398.
[DOI] -
37. Ni C, Wang K, Jin L, Liu Y, Chen J, Yang L, et al. Built-in electric field guides oxygen evolution electrocatalyst reconstruction. Chem Commun. 2025;61:658-668.
[DOI] -
38. Zhang Y, Tan X, Pei XY Wang Y, Yi S, Wang Q, et al. Multiple interface coupling triggered built-in electric field over double-sandwiched RGO/cobalt silicate/cobalt-iron phosphide for improving the overall water-splitting performance. Inorg Chem Front. 2025;12:2439-2452.
[DOI] -
39. Lin T, Yang H, Dong J, Ni C, Gao X, Li J, et al. Constructing Ni2P/CoP heterojunction with stable built-in electric field to boost overall water splitting at industrial current density. Fuel. 2025;396:135282.
[DOI] -
40. Yan L, Chen YH, Xie JC, Li H. Optimizing heterointerface of NiCoP-Co/MXene with regulated charge distribution via built-in electric field for efficient overall water-splitting. Rare Met. 2025;44:1067-1083.
[DOI] -
41. Sidra S, Hoa VH, Kim DH. d-band center upshift and electronic modulation of nickel cobalt phosphide integrated with reduced graphene oxide for stable and effcient water-splitting electrocatalysis. J Energy Chem. 2025;103:264-273.
[DOI] -
42. Ren K, Xu WJ, Li K, Cao JM, Gu ZY, Liu DH, et al. Br-induced d-band regulation on superhydrophilic isostructural cobalt phosphide for efficient overall water splitting. Adv Funct Mater. 2025;35:2415585.
[DOI] -
43. Chu X, Wang Y, Jing L, Jiang W, Wu Y, Lu M, et al. Design and construction of metal sulfide/phosphide heterostructures with optimized d-band center and boosted electrocatalytic oxygen evolution. J Mater Sci Technol. 2025;233:38-47.
[DOI] -
44. Xu T, Jiao D, Fan J, Dong Y, Jin Z, Zhang L, et al. “Similar stacking”-inspired compressive strain of heterogeneous phosphide for efficient hydrogenevolution. Carbon Energy 2025;
[DOI] -
45. Guo H, Pan L, Jiang H, Gao M, Wang H, Khan A, et al. Interface engineering of flower-like Co2P/WO3-x/carbon cloth catalysts with oxygen vacancies for efficient oxygen evolution reaction. Chem Eur J. 2025;31(2):e202402907.
[DOI] -
46. Kwong WL, Gracia-Espino E, Lee CC, Sandström R, Wågberg T, Messinger J. Cationic vacancy defects in iron phosphide: A promising route toward efficient and stable hydrogen evolution by electrochemical water splitting. ChemSusChem. 2017;10(22):4544-4551.
[DOI] -
47. Guo Z, Bi M, He H, Liu Z, Duan Y, Cao W. Defect engineering associated with cationic vacancies for promoting electrocatalytic water splitting in iron-doped Ni2P nanosheet arrays. J Coll Int Sci. 2024;654:785-794.
[DOI] -
48. Wei X, Jiao Y, Zou X, Guo Y, Li W, Ai T. P vacancy-induced electron redistribution and phase reconstruction of CoFeP for overall water splitting at industrial-level current density. Inorg Chem Front. 2025;12(7):2678-2690.
[DOI] -
49. Zhang F, Wang K, Zhang H, Yang S, Xu M, He Y, et al. Dynamic reconstruction of Ce-doped Fe2P/NiCoP hybrid for ampere-level oxygen evolution in anion exchange membrane water electrolysis. Adv Funct Mater. 2025;
[DOI] -
50. Wei X, Huang L, Yu Y, Sun D, Qu Y, Yuan X, et al. Crystalline CoP@ amorphous WP2 coaxial nanowire arrays as bifunctional electrocatalyst for water splitting. Small. 2025;21(17):2412689.
[DOI] -
51. Zhang J, Zhang Y, Zhou J, Guo H, Qi L. Electronic engineering of crystalline/amorphous CoP/FeCoPx nanoarrays for efficient water electrolysis. Small Methods. 2025;9(3):2401139.
[DOI] -
52. Chen X, Cheng Z, Li J, Chen H, Liu S, Wei S, et al. Achieving advanced hydrogen evolution under large current density using an amorphous/crystalline core-shell electrocatalyst of a-NiCoP/Co2P. Dalton Trans. 2025;54(7):2833-2841.
[DOI] -
53. Liu Y, Ji L, Xu D, Ye Q, Zhao Y, Cheng Y. Construction of crystalline/amorphous Ni2P/FePOx/graphene heterostructure by microwave irradiation for efficient oxygen evolution. J Colloid Interf Sci. 2025;683:474-483.
[DOI] -
54. Fu X, Liao H, Zhang Z, Zheng Y, Lu J, Cheng S, et al. Medium-entropy heterostructure of crystalline NiCoFeP@ amorphous NiCoFe-LDH for industrial-current density and ultrastable overall water splitting. Chem Eng J. 2025;505:159520.
[DOI] -
55. Hu Y, Shen B, Liu W, Pan Y, Huang J, Zhu X, et al. Activation of lattice oxygen by loading NiFeP nanoparticles on nitrogen-doped porous carbon to enhance OER activity. J Alloys Compd. 2025;1010:178251.
[DOI] -
56. Huang T, Xu G, Ding H, Liu X, Zhang L. NiFeP nanosheet arrays supported on nickel nitrogen codoped carbon nanofiber as an efficient bifunctional catalyst for overall water splitting. Sep Purif Technol. 2025;354:129094.
[DOI] -
57. Zuo G, Wang H, Yan H, Li Z, Zhao P, Wang C, et al Cobalt phosphide@S-doped carbon nanobelt arrays derived from metal-organic framework as an efficient bifunctional electrocatalyst for water splitting. J Coll Interf Sci 2025;
[DOI] -
58. Chen N, Che S, Zhang Y, Li H, Li Y, He X. Size effect of carbon nanotubes confined CoP-Ni2P heterostructure via phosphorylation-induction strategy for efficient overall water splitting. Rare Met. 2025;44:4740-4755.
[DOI] -
59. Liu Z, Feng C, Yang S, Li K, Huang Z, Sun D. 1D/3D dual carbon-supported Mott-Schottky-type Co-Co2P heterojunctions for pH-universal hydrogen evolution. J Coll Int Sci. 2024;657:559-566.
[DOI] -
60. Li W, Liu J, Guo P, Li H, Fei B, Guo Y, et al. Co/CoP heterojunction on hierarchically ordered porous carbon as a highly efficient electrocatalyst for hydrogen and oxygen evolution. Adv Energy Mater. 2021;11(42):2102134.
[DOI] -
61. Li J, Hu J, Zhang M, Gou W, Zhang S, Chen Z, et al. A fundamental viewpoint on the hydrogen spillover phenomenon of electrocatalytic hydrogen evolution. Nat Commun. 2021;12:3502.
[DOI] -
62. He C, Liu Q, Wang H, Xia C, Li FM, Guo W, et al. Regulating reversible oxygen electrocatalysis by built-in electric field of heterojunction electrocatalyst with modified d-band. Small. 2023;19(15):2207474.
[DOI] -
63. Wang G, Zou H, Zhang H, Xiang J, Yang M, Liu J. Carbon-supported FeCoNiP/FeCoNi Mott-Schottky electrocatalyst for alkaline oxygen evolution reaction. Electrochim Acta. 2023;462:142723.
[DOI] -
64. Kong D, Xu Q, Chu N, Wang H, Lim YV, Cheng J, et al. Rational construction of 3D self-supported MOF-derived cobalt phosphide-based hollow nanowall arrays for efficient overall water splitting at large current density. Small. 2024;20(27):2310012.
[DOI] -
65. Maślana K, Chen X, Mijowska E. Highly active and robust electrocatalyst based on nickel phosphide supported by borophene for oxygen evolution reaction. ACS Appl Energy Mater. 2024;7(19):8321-8332.
[DOI] -
66. Wu J, Wang ZF, Guan T, Zhang G, Zhang J, Han J, et al. Optimizing band structure of CoP nanoparticles via rich‐defect carbon shell toward bifunctional electrocatalysts for overall water splitting. Carbon Energy. 2023;5(3):e268.
[DOI] -
67. Hua Y, Xu Q, Hu Y, Jiang H, Li C. Interface-strengthened CoP nanosheet array with Co2P nanoparticles as efficient electrocatalysts for overall water splitting. J Energy Chem. 2019;37:1-6.
[DOI] -
68. Yu X, Wang X, He P, Yang G, Gao L, Qin F, et al. Electronic modulation on allotropic Co2P/CoP heterojunctions with N, P co-doped porous carbon matrix through metal-injection toward high-efficiency water splitting. Appl Surf Sci. 2024;670:160665.
[DOI] -
69. Xu H, Jin L, Wang K, Yang L, Liu Y, He G, et al. Interfacial built-in electric fields facilitating surface reconstruction in heterojunction electrocatalysts for boosting water oxidation and simulated seawater oxidation. Fuel. 2024;369:131716.
[DOI] -
70. Wang J, Yan M, Wang S, Liu Z, Zhang L, Xu Y, et al. Electron density regulation of bimetal phosphates via heterojunction interface engineering for electrocatalytic reaction. Colloids Surf A Physicochem En. Asp. 2024;683:132989.
[DOI] -
71. Deng SQ, Pei MJ, Zhao ZH, Wang KL, Zheng H, Zheng SR, et al. Metal-organic framework derived heterostructured phosphide bifunctional electrocatalyst for efficient overall water splitting. J Coll Interf Sci. 2024;676:884-895.
[DOI] -
72. Li Y, Yu X, Gao J, Ma Y. Hierarchical Ni2P/Zn-Ni-P nanosheet array for efficient energy-saving hydrogen evolution and hydrazine oxidation. J Mater Chem A. 2023;11(5):2191-2202.
[DOI] -
73. Zhang L, Shi X, Xu A, Zhong W, Zhang J, Shen S. Novel CoP/CoMoP2 heterojunction with nanoporous structure as an efficient electrocatalyst for hydrogen evolution. Nano Res. 2024;17:3693-3699.
[DOI] -
74. Zhi L, Zhang M, Tu J, Li M, Liu J. Coordination polymer and layered double hydroxide dual-precursors derived polymetallic phosphides confined in N-doped hierarchical porous carbon nanoflower as a highly efficient bifunctional electrocatalyst for overall water splitting. J Coll Interf Sci. 2024;659:82-93.
[DOI] -
75. Zhang P, Man H, Huo Y, Zhang F, Liu Y, Wu R, et al. CrOx modified nickel phosphides electrocatalyst for stable hydrogen evolution reaction in neutral media. Adv Funct Mater. 2024;34(49):2409365.
[DOI] -
76. Hu X, Zhang S, Sun J, Yu L, Qian X, Hu R, et al. 2D Fe-containing cobalt phosphide/cobalt oxide lateral heterostructure with enhanced activity for oxygen evolution reaction. Nano Energy. 2019;56:109-117.
[DOI] -
77. Bhanja P, Kim Y, Paul B, Kaneti YV, Alothman AA, Bhaumik A, et al. Microporous nickel phosphonate derived heteroatom doped nickel oxide and nickel phosphide: Efficient electrocatalysts for oxygen evolution reaction. Chem Eng J. 2021;405:126803.
[DOI] -
78. Wang M, Zhang C, Meng T, Pu Z, Jin H, He D, et al. Iron oxide and phosphide encapsulated within N,P-doped microporous carbon nanofibers as advanced tri-functional electrocatalyst toward oxygen reduction/evolution and hydrogen evolution reactions and zinc-air batteries. J Power Sources. 2019;413:367-375.
[DOI] -
79. Zhang L, Lei Y, Xu W, Wang D, Zhao Y, Chen W, et al. Highly active and durable nitrogen-doped CoP/CeO2 nanowire heterostructures for overall water splitting. Chem Eng J. 2023;460:141119.
[DOI] -
80. Zhang T, Ren X, Ma F, Jiang X, Wen Y, He W, et al. MOF-derived Co(Ni)Ox species loading on two-dimensional cobalt phosphide: A Janus electrocatalyst toward efficient and stable overall water splitting. Appl Mater Today. 2023;34:101912.
[DOI] -
81. Jang GY, Kim S, Choi J, Park J, An SE, Baek J, et al. Bulk-heterojunction electrocatalysts in confined geometry boosting stable, acid/alkaline-universal water electrolysis. Adv Energy Mater. 2024;14:2303924.
[DOI] -
82. Ren JT, Chen L, Wang HY, Tian WW, Song XL, Kong QH, et al. Synergistic activation of crystalline Ni2P and amorphous NiMoOx for efficient water splitting at high current densities. ACS Catal. 2023;13(14):9792-9805.
[DOI] -
83. Hu J, Yin J, Peng A, Zeng D, Ke J, Liu J, et al. In situ hydroxide growth over nickel-iron phosphide with enhanced overall water splitting performances. Small. 2024;20(44):2402881.
[DOI] -
84. Yang HM, Wang HY, Sun ML, Yuan ZY. Interface engineering of bifunctional nickel hydroxide/ nickel phosphide heterostructure for efficient intermittent hydrazine-assisted water splitting. Chem Eng J. 2023;475:146134.
[DOI] -
85. Shahzad U, Saeed M, Marwani HM, Al-Humaidi JY, Rehman S, Althomali RH, et al. Transition metal-based chalcogenides as electrocatalysts for overall water splitting in hydrogen energy production. Int J Hydrogen Energy. 2024;65:215-224.
[DOI] -
86. Qian Y, Zhang F, Luo X, Zhong Y, Kang DJ, Hu Y. Synthesis and electrocatalytic applications of layer-structured metal chalcogenides composites. Small. 2024;20(26):2310526.
[DOI] -
87. Bao W, Liu J, Ai T, Han J, Hou J, Li W, et al. Unveiling the role of surface self-reconstruction of metal chalcogenides on electrocatalytic oxygen evolution reaction. Adv Funct Mater. 2024;34(48):2408364.
[DOI] -
88. Qian Y, Sun Y, Zhang F, Luo X, Li K, Shen L, et al. In situ construction of layered transition metal phosphides/sulfides heterostructures for efficient hydrogen evolution in acidic and alkaline media. Chem Eng J. 2024;490:151693.
[DOI] -
89. Liu S, Xuan H, Song Z, Meng L, Wang J, Liang X, et al. Interface engineering of multi-component phosphide/sulfide core-shell heterostructure for efficient overall water splitting. Int J Hydrogen Energy. 2024;59:1106-1114.
[DOI] -
90. Ali A, Long F, Shen PK. Innovative strategies for overall water splitting using nanostructured transition metal electrocatalysts. Electrochem Energy Rev. 2022;5:1.
[DOI] -
91. Li M, Zheng K, Zhang J, Li X, Xu C. Design and construction of 2D/2D sheet-on-sheet transition metal sulfide/phosphide heterostructure for efficient oxygen evolution reaction. Appl Surf Sci. 2021;565:150510.
[DOI] -
92. Huang KM, Wu JJ. Bifunctional nickel phosphide nanoparticle/nickel cobalt sulfide nanosheet framework for electrocatalytic simultaneous hydrogen evolution and 2,5-Furandicaroxylic acid production. Chem Eng J. 2024;484:149772.
[DOI] -
93. Wang HY, Ren JT, Wang L, Sun ML, Yang HM, Lv XW, et al. Synergistically enhanced activity and stability of bifunctional nickel phosphide/sulfide heterointerface electrodes for direct alkaline seawater electrolysis. J Energy Chem. 2022;75:66-73.
[DOI] -
94. Ren JT, Chen L, Wang HY, Tian WW, Zhai SX, Feng Y, et al. Modulating interfacial charge distribution of Ni2P-NiSe2 by multiple interface engineering for accelerating water splitting with industry-level activity and stability. Appl Catal B Environ Energy. 2024;347:123817.
[DOI] -
95. Surendranath Y, Kanan MW, Nocera DG. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J Am Chem Soc. 2010;132(46):16501-16509.
[DOI] -
96. Kanan MW, Nocera DG. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science. 2008;321(5892):1072-1075.
[DOI] -
97. Kanan MW, Surendranath Y, Nocera DG, Cobalt-phosphate oxygen-evolving compound. Chem Soc Rev 2009;
[DOI] -
98. Li W, Chen M, Lu Y, Qi P, Liu G, Zhao Y, et al. One-pot electrodeposition synthesis of NiFe-phosphate/phosphide hybrid nanosheet arrays for efficient water splitting. Appl Surf Sci. 2022;598:153717.
[DOI] -
99. Chen Z, Zeng X, Li X, Lv Z, Li J, Zhang Y. Strong metal phosphide-phosphate support interaction for enhanced non-noble metal catalysis. Adv Mater. 2022;34:2106724.
[DOI] -
100. Thakur N, Kumar M, Mandal D, Nagaiah TC. Nickel iron phosphide/phosphate as an oxygen bifunctional electrocatalyst for high-power-density rechargeable Zn-air batteries. ACS Appl Mater Interfaces. 2021;13(44):52487-52497.
[DOI] -
101. He L, Gong L, Gao M, Yang CW, Sheng GP. In situ formation of NiCoP@phosphate nanocages as an efficient bifunctional electrocatalyst for overall water splitting. Electrochim Acta. 2020;337:135799.
[DOI] -
102. Zhao J, Zhang Y, Xia Y, Zhang B, Du Y, Song B, et al. Strong phosphide-metaphosphate interaction in RuP/CoNiP4O12 for enhanced electrocatalytic water splitting. Appl Catal B Environ. 2023;328:122447.
[DOI] -
103. Hoa VH, Austeria M, Dao HT, Mai M, Kim DH. Dual-phase cobalt phosphide/phosphate hybrid interactions via iridium nanocluster interfacial engineering toward efficient overall seawater splitting. Appl Catal B Environ. 2023;327:122467.
[DOI] -
104. Liang Y, Zhao X, Yan P, Xue L, Li H, Gu L, et al. Crystalline Ni5P4/amorphous CePO4 core/shell heterostructure arrays for highly-efficient electrocatalytic overall water splitting. J Coll Interf Sci. 2024;655:565-575.
[DOI] -
105. Fan J, Wang L, Xiang X, Liu Y, Shi NE, Lin Y, et al. Porous flower-like nanoarchitectures derived from nickel phosphide nanocrystals anchored on amorphous vanadium phosphate nanosheet nanohybrids for superior overall water splitting. Small Methods. 2024;8:2301279.
[DOI] -
106. Li Y, Yin Z, Liu X, Cui M, Chen S, Ma T. Current progress of molybdenum carbide-based materials for electrocatalysis: potential electrocatalysts with diverse applications. Mater Today Chem. 2021;19:100411.
[DOI] -
107. Yan X, Deng D, Wu S, Li H, Xu L. Development of transition metal nitrides as oxygen and hydrogen electrocatalysts. Chin J Struct Chem. 2022;41:2207004-2207015.
[DOI] -
108. Chen P, Ye J, Wang H, Ouyang L, Zhu M. Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting. J Alloys Compd. 2021;883:160833.
[DOI] -
109. Hu Y, Yang H, Chen J, Xiong T, Balogun MSJT, Tong Y. Efficient hydrogen evolution activity and overall water splitting of metallic Co4N nanowires through tunable d-orbitals with ultrafast incorporation of FeOOH. ACS Appl Mater Interfaces. 2019;11:5152-5158.
[DOI] -
110. Gao Q, Zhang W, Shi Z, Yang L, Tang Y. Structural design and electronic modulation of transition metal-carbide electrocatalysts toward efficient hydrogen evolution. Adv Mater. 2018;31(2):1802880.
[DOI] -
111. Liu A, Liang X, Ren X, Guan W, Gao M, Yang Y, et al. Recent progress in MXene-based materials: potential high-performance electrocatalysts. Adv Funct Mater. 2020;30(38):2003437.
[DOI] -
112. Jiang J, Sun R, Huang X, Xu W, Zhou S, Wei Y, et al. In-situ derived Mo-doped NiCoP and MXene to form Mott-Schottky heterojunction with tunable surface electron density to promote overall water splitting. Compos Part B Eng. 2023;263:110834.
[DOI] -
113. Cai Z, Xu L, Zhou Y, Gao L, An X, Ma X, et al. Fabrication of cobalt phosphide/nitride/carbon nanotube composite: An efficient bifunctional catalyst for hydrogen and oxygen evolution. Int J Hydrogen Energy. 2024;82:559-566.
[DOI] -
114. Gu Y, Wu A, Jiao Y, Zheng H, Wang X, Xie Y, et al. Two-dimensional porous molybdenum phosphide/nitride heterojunction nanosheets for pH-universal hydrogen evolution reaction. Angew Chem Int Ed. 2021;60:6673-6681.
[DOI] -
115. Ma W, Li D, Liao L, Zhou H, Zhang F, Zhou X, et al. High-performance bifunctional porous iron-rich phosphide/nickel nitride heterostructures for alkaline seawater splitting. Small. 2023;19:2207082.
[DOI] -
116. Qin M, Chen L, Zhang H, Humayun M, Fu Y, Xu X, et al. Achieving highly efficient pH-universal hydrogen evolution by Mott-Schottky heterojunction of Co2P/Co4N. Chem Eng J. 2023;454:140230.
[DOI] -
117. Zhou X, Mo Y, Yu F, Liao L, Yong X, Zhang F, et al. Engineering active iron sites on nanoporous bimetal phosphide/nitride heterostructure array enabling robust overall water splitting. Adv Funct Mater. 2023;33(6):2209465.
[DOI] -
118. Liu B, Zhao P, Wu Z, Liu C, Jing H, Song J, et al. Prussian blue analogue-derived CoP nanocubes supported on MXene toward an efficient bifunctional electrode with enhanced overall water splitting. J Coll Interf Sci. 2024;661:709-719.
[DOI] -
119. Liao L, Zhou Q, Liu F, Ma Y, Cheng C, Huang H, et al. Deciphering the in situ reconstruction of metal phosphide/nitride dual heterostructures for robust alkaline hydrogen evolution above 3 A cm−2. Small. 2024;20:2311289.
[DOI] -
120. Du H, Du Z, Wang T, He S, Yang K, Wang K, et al. Interface engineering of tungsten carbide/phosphide heterostructures anchored on N,P-codoped carbon for high-efficiency hydrogen evolution reaction. Sci China Mater. 2022;65:967-973.
[DOI] -
121. Chu C, Liu J, Wei L, Feng J, Li H, Shen J. Iron carbide and iron phosphide embedded N-doped porous carbon derived from biomass as oxygen reduction reaction catalyst for microbial fuel cell. Int J Hydrogen Energy. 2023;48:4492-4502.
[DOI] -
122. Wu Y, Xiao Z, Jin Z, Li X, Chen Y. The cobalt carbide/bimetallic CoFe phosphide dispersed on carbon nanospheres as advanced bifunctional electrocatalysts for the ORR, OER, and rechargeable Zn-air batteries. J Coll Interf Sci. 2021;590:321-329.
[DOI] -
123. Wei P, Sun X, Wang M, Xu J, He Z, Li X, et al. Construction of an N-decorated carbon-encapsulated W2C/WP heterostructure as an efficient electrocatalyst for hydrogen evolution in both alkaline and acidic media. ACS Appl Mater Interfaces. 2021;13(45):53955-53964.
[DOI] -
124. Kulkarni R, Lingamdinne LP, Koduru JR, Karri RR, Chang YY, Kailasa SK, et al. Recent advanced developments and prospects of surface functionalized MXenes-based hybrid composites toward electrochemical water splitting applications. ACS Materials Lett. 2024;6(7):2660-2686.
[DOI] -
125. Kim JY, Roh SH, Xia C, Sim UK, Kim JK. MXene-based hybrid materials for electrochemical and photoelectrochemical H2 generation. J Energy Chem. 2024;93:111-125.
[DOI] -
126. Liu S, Lin Z, Wan R, Liu Y, Liu Z, Zhang S, et al. Cobalt phosphide supported by two-dimensional molybdenum carbide (MXene) for the hydrogen evolution reaction, oxygen evolution reaction, and overall water splitting. J Mater Chem A. 2021;9:21259-21269.
[DOI] -
127. Jin C, Peng H, Zeng X, Liu Z, Ding D. Hierarchical assembly of NiFe-PB-derived bimetallic phosphides on 3D Ti3C2 MXene ribbon networks for efficient oxygen evolution. ChemPhysMater. 2024;3:118-124.
[DOI] -
128. Su H, Jin C, Zhang X, Yu Z, Zeng X. Recent progress in the synthesis and electrocatalytic application of MXene-based metal phosphide composites. Carbon Neutraliz. 2024;3(6):1009-1035.
[DOI] -
129. Zhang J, Wang X, Du F, Wu J, Xiao S, Zhou Y, et al. Phosphorous vacancy and built-in electric field effect of Co-doped MoP@MXene heterostructures to tune catalytic activity for efficient overall water splitting. Small. 2024;20(42):2400304.
[DOI] -
130. Jeong M, Park S, Kwon T, Kwon M, Yuk S, Kim S, et al. Interface engineering via Ti3C2Tx MXene enabled highly efficient bifunctional NiCoP array catalysts for alkaline water splitting. ACS Appl Mater Interfaces 2024. 16(27):34798-34808;
[DOI] -
131. Ma W, Qiu Z, Li J, Hu L, Li Q, Lv X, et al. Interfacial electronic coupling of V-doped Co2P with high-entropy MXene reduces kinetic energy barrier for efficient overall water splitting. J Energy Chem. 2023;85:301-309.
[DOI] -
132. Ma W, Fei J, Wang J, Dai Y, Hu L, Lv X, et al. Coupling of modulating d-band center and optimizing interface electronic structure via MXene and Bi-metal doping in CoP achieving efficient bifunctional catalytic activity for water splitting. Sep Purif Technol. 2024;351:128136.
[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




