Abstract
Green hydrogen produced through photocatalytic water splitting is pivotal for achieving carbon neutrality and facilitating the transition to carbon-free energy conversion systems. Although photocatalytic systems have demonstrated high activity and operational safety at the laboratory scale, their large-scale application for practical hydrogen production remains limited by the long-term stability and performance of photocatalysts, as well as the complexity and safety concerns associated with scaling up photocatalytic reaction platforms. Meeting these requirements would establish a targeted framework for advancing photolysis technology and accelerating the transition from fundamental research to industrial-scale implementation of photocatalytic hydrogen generation. This perspective highlights the fundamental principles for improving photocatalysis and explores diverse device configurations for large-scale hydrogen production, while outlining the critical prerequisites for both photocatalytic materials and reactor architectures, thereby paving the way for future commercialization.
Graphical Abstract
Keywords
1. Introduction
Given the escalating environmental pollution and the growing global energy demand, it is urgent to develop a promising energy carrier that can reduce reliance on traditional fossil fuels while enabling the transition to carbon-free energy conversion systems[1-3]. Hydrogen, an environmentally friendly, pollution-free energy carrier with high energy density, has therefore attracted considerable attention. There are two main strategies for producing high-purity green hydrogen: (i) electrocatalytic water splitting, in which electricity generated by solar panels drives an electrolytic cell to produce hydrogen, and (ii) photocatalytic water splitting, in which solar energy directly powers photolytic reactions to generate hydrogen. Compared with conventional hydrogen production methods that result in high carbon emissions, such as natural gas reforming and coal gasification, electro- and photocatalytic hydrolysis driven by renewable energy offer a promising pathway for producing high-purity H2 while simultaneously mitigating environmental pollution[4,5].
There are notable differences between photocatalytic and electrocatalytic hydrogen production, one of which lies in the complexity of catalytic system configurations. Electrochemical water splitting requires an expensive photovoltaic (PV) array to capture solar energy and a battery system for electricity storage, involving a complex process that first converts solar energy into electricity and then into chemical energy. In contrast, photocatalytic water splitting offers a significant advantage through a simpler process that directly converts solar energy into chemical energy[6], thereby eliminating intermediate energy conversion steps and reducing associated energy losses. rom a cost perspective, photocatalytic systems typically employ non-precious metal catalysts and inexpensive reactor materials, whereas electrocatalytic systems rely on costly PV panels, batteries, and electrode materials, making photocatalysis a more cost-effective pathway. However, catalytic performance remains a critical factor in determining the feasibility of large-scale hydrogen production. Electrocatalysis, combining photovoltaics and water electrolysis, is comparatively mature and can achieve solar-to-hydrogen (STH) conversion efficiencies of up to 30%[7]. By contrast, the STH efficiency of direct photocatalytic water splitting remains below 1%[8], reflecting a substantial performance gap relative to electrocatalysis. Overall, photocatalysis offers distinct advantages for hydrogen production, including simpler system architecture and lower capital costs, as it avoids the need for expensive PV arrays and battery storage systems. Nevertheless, the primary barrier to its large-scale deployment is the insufficient catalytic performance. The STH efficiency of current photocatalytic systems remains below 1%, far lower than the 30% achieved by electrocatalysis, and this large efficiency gap represents the principal bottleneck hindering the commercial viability of photocatalytic hydrogen production.
2. Optimized Design for Photocatalysts
From the perspective of semiconductor photochemistry, photocatalysis is a continuous process in which photoinduced charges generated under light irradiation initiate or accelerate a series of redox reactions (Figure 1a), such as the hydrogen evolution reaction (HER) and oxygen evolution reaction in water splitting, as well as carbon dioxide and nitride reduction reactions[9]. Several factors influence photocatalytic performance, including limited light-harvesting capacity, slow charge separation kinetics in both the bulk and surface phases, and ultimately poor redox capability[10]. Among these, a major challenge is the high charge recombination rate in the bulk and on the surface due to Coulomb interactions, which has become the primary limiting factor for photocatalytic efficiency[11]. On the one hand, the slow kinetics of bulk charges, which are influenced by temperature, concentration, and effective mass, require hundreds of picoseconds for charges to migrate to the surface, whereas bulk charge recombination occurs within only a few picoseconds. Similarly, while surface charges that initiate redox reactions persist for several microseconds, surface charge recombination takes place within just tens of nanoseconds[12]. Clearly, the rate of charge recombination is several orders of magnitude higher than that of charge separation, resulting in substantial energy loss and reduced photocatalytic performance. One effective strategy to suppress bulk charge recombination is the use of ferroelectric photocatalysts. The displacement of positive and negative centers in these materials induces inherent surface polarization and generates a polarization electric field strong enough to facilitate bulk charge separation[13]. For surface charge recombination, surface asymmetry induced by surface modification can alter the electronic structure and trigger the formation of electron accumulation and depletion regions, thereby enriching reactive sites and promoting local surface charge separation[14,15]. This polarization effect, termed surface polarization, can be complemented by piezoelectric and ferroelectric polarization, both of which also effectively enhance surface charge separation[16]. Another key challenge lies in the incompatibility between light absorption and redox capability in single-component photocatalysts (Figure 1b)[17]. According to photochemistry theory, broad light absorption requires a narrow bandgap, whereas strong redox capability demands a more positive valence band (VB) and a more negative conduction band (CB), corresponding to a wide bandgap. It is therefore inherently impossible to maximize both properties simultaneously within a single-component photocatalyst.
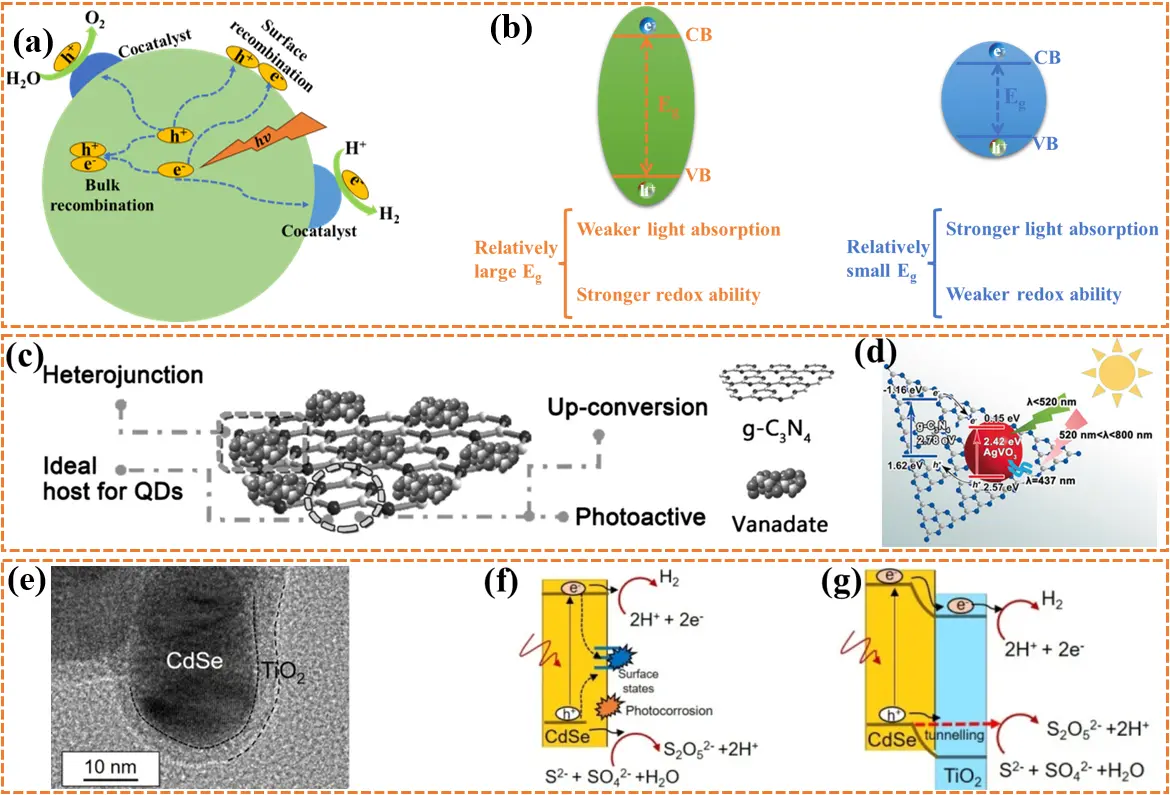
Figure 1. (a) The photocatalytic reaction mechanism; (b) The incompatibility between strong redox and absorption ability within single-component photocatalysts; (c) The synthesis method of AgVO3/g-C3N4 heterojunction; (d) The photocatalytic principle of AgVO3/g-C3N4 heterojunction. Republished with permission from[28]; (e) High resolution TEM images of TiO2/CdSe heterostructure; Possible reaction mechanism of (f) CdSe and (g) TiO2/CdSe heterostructure. Republished with permission from[34]. QD: quantum dot; VB: valence band; CB: conduction band.
Based on the challenges described above, various modification strategies have been developed to enhance the photocatalytic performance of single-component photocatalysts, including defect engineering[18,19], element doping[20,21], morphology control[22,23], and constructing heterojunctions by integrating other metals or semiconductors[24,25]. Among these strategies, heterojunction construction has emerged as one of the most promising approaches to overcome these limitations due to its unique synergistic effects. A heterojunction is an interface composed of different photocatalysts with mismatched bandgap alignments, which not only broadens the light absorption range but also optimizes the redox capability to match the required redox potentials[26]. Moreover, heterojunction formation can reduce bulk charge recombination by inducing a built-in electric field (BIEF), which promotes charge separation at the heterointerface through the continuous flow of charges driven by differences in work functions[27]. For instance, an innovative 0D/2D heterojunction was proposed to enhance visible-light response and bulk charge separation[28]. In this system, graphitic carbon nitride nanosheets (g-C3N4 NPs) with a negative zeta potential absorb Ag+ ions via electrostatic interactions and coordinate them at nitrogen sites within tri-s-triazine units, followed by the in-situ formation of AgVO3 quantum dots upon addition of NH4VO3 (Figure 1c). The resulting AgVO3/g-C3N4 heterojunction exhibited significantly enhanced visible-light absorption and accelerated bulk charge separation compared with pure AgVO3 and g-C3N4, owing to the distinct energy band alignment and the BIEF at the heterointerface that facilitate charge transfer (Figure 1d).
For photocatalyst systems applied in large-scale hydrogen production, two excitation schemes are commonly employed: one-step and two-step excitation systems. In one-step excitation systems, a single semiconductor photocatalyst is used for water splitting. Specifically, when the energy of incident light is equal to or greater than the semiconductor’s bandgap, photogenerated electrons are excited to the CB of the photocatalyst, leaving an equal number of photogenerated holes in the VB. Due to Coulombic attraction, these photoinduced charges tend to recombine, resulting in the loss of charge carriers[29]. The remaining charges that avoid recombination migrate to the photocatalyst surface and participate in redox reactions with other species. In two-step excitation systems, two semiconductor photocatalysts are employed: one as an O2-evolution photocatalyst with a more positive VB potential, and the other as an H2-evolution photocatalyst with a more negative CB potential, allowing both to maintain stronger redox abilities. This mechanism resembles the traditional Z-scheme heterojunction, where redox pairs facilitate electron transfer. The photoinduced charges at lower potentials are transferred between the O2- and H2-evolution photocatalysts via the redox pairs, achieving spatial separation of charges. A functional membrane, such as a proton exchange membrane (PEM), plays a crucial role in separating gas mixtures by transferring protons, thereby enabling the generation of different gases in separate chambers. This configuration also effectively reduces the risk of explosion from mixed oxyhydrogen gas. While two-step excitation systems generally achieve higher STH efficiencies by minimizing charge recombination, a major challenge remains the mismatch of energy band configurations between the O2- and H2-evolution photocatalysts.
In addition to promoting effective charge separation, heterojunction construction also holds promise for active site modification, similar to heteroatom doping or defect engineering that alters local electronic density[30]. Taking heterojunction as a representative example, their formation induces a continuous electronic flow due to differences in Fermi levels, accompanied by changes in interfacial electron structures and coordination environments. These changes can directly affect the p-band center, thereby modulating the absorption and desorption behaviors of reaction intermediates and the energy barriers of specific reaction steps. Furthermore, the interfacial electronic flow facilitates the simultaneous formation of electron accumulation and depletion regions, generating active sites with dual functionalities. This effectively overcomes the challenge of certain reaction intermediates encountering excessively high energy barriers on a single-component photocatalyst, thereby smoothing the overall reaction pathway. For photocatalytic H2 production, the primary reaction steps include proton absorption and H2 desorption, which are typically difficult to enhance simultaneously within a single photocatalyst[31]. Interfacial electronic interactions can precisely modulate the electronic density at active sites to lower the energy barrier of H2 desorption or generate new active sites. For example, the NH2-MIL-125(Ti)/Zn0.5Cd0.5S/NiS system was designed to promote H2 desorption[32], and the ZnCdS/NiCo2O4 heterostructure was constructed to facilitate the formation of additional active sites[33].
In addition to photocatalytic performance, the stability of photocatalysts is also a critical factor influencing large-scale hydrogen production. Stability requires the photocatalyst to maintain its physicochemical and structural integrity under prolonged UV irradiation and fluctuating temperatures, which is primarily affected by photocorrosion and surface fouling[34]. To address catalyst deactivation, as observed in materials such as CdS and ZnS, strategies including surface modification and protective coatings can be employed. For example, a TiO2-coated CdSe core-shell structure was designed to mitigate photocorrosion of CdSe during prolonged illumination (Figure 1e)[35]. Owing to the synergistic effects of the Type-II heterojunction and the protective TiO2 shell, the nanocapsule-shaped TiO2/CdSe heterostructure demonstrates superior hydrogen evolution performance, promoting efficient charge separation while physically shielding the CdSe core from photocorrosion (Figure 1f,g). Surface fouling caused by the accumulation of by-products and contaminants, particularly in the presence of sacrificial agents, blocks active sites and impedes reactions, directly compromising the stability of the photocatalyst. This issue can be effectively mitigated through periodic gentle acid washing, which restores active sites and extends the operational lifetime of the photocatalyst. However, acid washing may also lead to a decline in photocatalytic performance due to the introduction of new defects or impurities, surface passivation, chemical dissolution, or structural damage[36-38]. Therefore, exploring alternative strategies to mitigate surface fouling is important for enhancing photocatalytic stability in large-scale production. These strategies include photocatalyst surface design with self-cleaning properties, synthesis of multi-level nanostructures, and rational construction of photocatalytic reactors. Photocatalysts with self-cleaning surfaces are particularly promising, as they can degrade contaminants on their surfaces through intrinsic photocatalytic activity or facilitate contaminant removal via water flow by forming special surface wettability, such as superhydrophilicity, superhydrophobicity, or super-amphiphilicity[39,40]. For photocatalysts with multi-level nanostructures, these special structures provide higher specific surface area and more active sites while reducing the risk of channel blockage. Moreover, enhanced mass transfer promotes rapid bubble escape, generating a flushing effect that prevents pollutant deposition and facilitates desorption of adsorbed species[41]. In photocatalytic reactors, liquid flow is crucial. Continuous liquid movement generates impact forces that prevent static deposition of pollutants on the catalyst surface and enable continuous product separation, keeping the photocatalyst surface clean[42].
3. Photolysis Device Construction
The challenges limiting the intrinsic activity of photocatalytic materials, such as visible-light harvesting capacity, charge recombination, and redox capability, can be addressed through various modification strategies at the laboratory scale[43]. Nevertheless, the practical application of photocatalytic materials in device fabrication, as well as the maintenance of stability and catalytic activity under real conditions, remains a significant challenge for large-scale hydrogen production. In 2015, an innovative large-scale reaction device with a total irradiation area of 0.756 m2 was developed. In this system, platinum-loaded mesoporous g-C3N4 photocatalysts were immobilized on stainless-steel plates using Nafion as a polymeric binder to prevent sedimentation and detachment, while triethanolamine was added as a sacrificial agent to maintain catalytic activity (Figure 2a)[44]. The total hydrogen yield from this device reached 18.2 L over 30 days. Simultaneously, a total of 83.8 kWh of energy was collected by the light sensor, corresponding to a hydrogen evolution rate of 0.22 L kWh-1, approximately 13% higher than laboratory-scale rates. Based on this rate, the calculated STH conversion efficiency was only 0.12%. Although triethanolamine effectively suppresses bulk charge recombination and prevents byproduct accumulation on reactive sites, its use may lead to water quality deterioration in real water bodies, representing an inherent limitation of this configuration. A larger-scale photocatalytic device was also designed, incorporating an arrayed panel reactor with a total area of 100 m2 using Al-doped SrTiO3 as the photocatalysts [45]. Prior to large-scale deployment, an indoor test using a small panel reactor was conducted under simulated solar light to activate the photocatalyst, achieving an STH conversion efficiency of 0.48%. Furthermore, the humid oxyhydrogen gas generated in the reactors naturally diffuses upward due to the reactor’s inclined structure and is separated through a hydrogen-permeable filter made of polyimide hollow fibers, effectively reducing the risk of explosion from mixed oxyhydrogen gas.
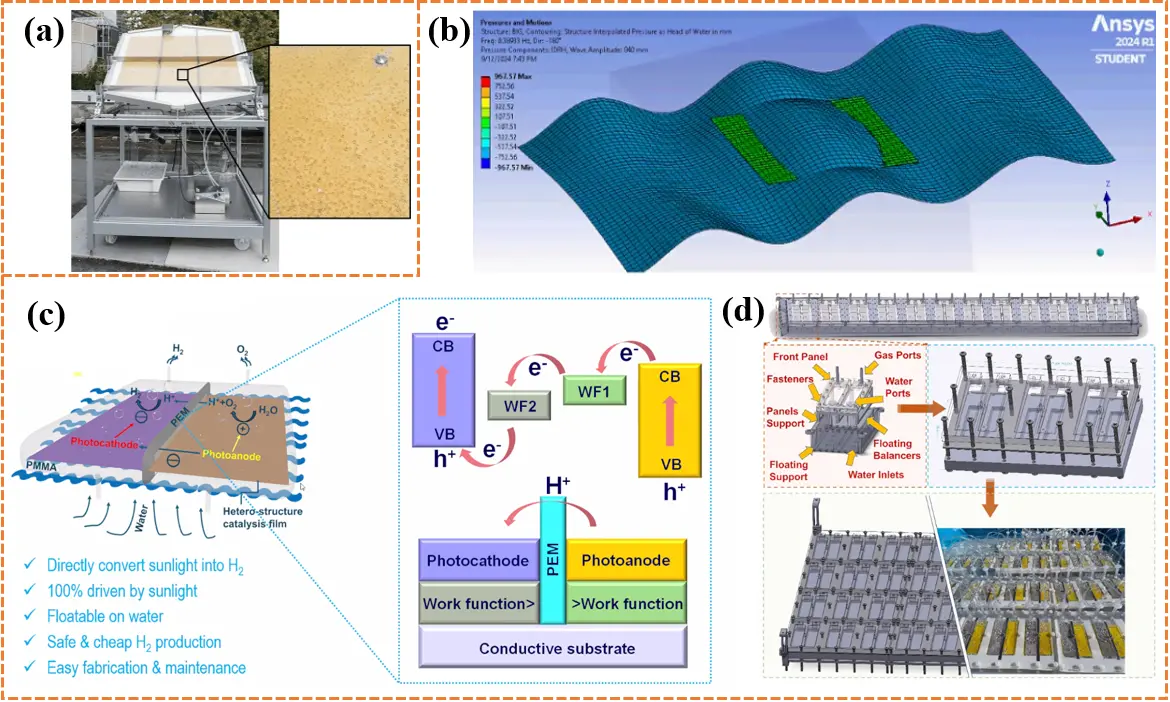
Figure 2. (a) The schematic diagram of the operating state of a large-scale photocatalytic hydrogen production reactor based on natural light. The high-magnification image shows the nucleation and release process of hydrogen bubbles on the surface of the supported mesoporous carbon nitride photocatalyst. Republished with permission from[44]; (b) Computational fluid dynamics modeling of a dual-chamber photoreactor for moving seawater[49]; (c) Schematic diagram of a photocatalytic water splitting device and the corresponding band alignment of the photocatalytic materia[49]; (d) The schematic diagram over the internal components and gas separation mechanism of a single dual-chamber reactor, accompanied by a photograph of the actual device. The schematic diagram and photograph of a larger-scale configuration at the bottom, which is assembled by linking multiple individual dual-chamber reactors in series or parallel[49]. VB: valence band; CB: conduction band.
Various large-scale photocatalytic reaction devices have been developed to improve upon the initial designs. Examples include the “Hydrogen Farm”, which employs a tandem proton capture-electron capture configuration for separated H2 and O2 production[12]; floatable photocatalytic platforms constructed from elastomer-hydrogel nanocomposites for hydrogen production via seawater splitting; and systems for the photoreforming of plastic wastes[46]. Despite these achievements, several inherent challenges continue to hinder large-scale photocatalytic H2 production[47,48]. These challenges include: (i) overall water splitting in a photocatalytic device produces a mixture of H2 and O2, raising safety concerns regarding potential explosions; (ii) the use of liquid mediators, such as sacrificial agents or organic additives, may cause pollution in real water environments, limiting large-scale deployment; (iii) no practical active floating device has yet been developed to overcome the limitations of land-based systems; and (iv) competitive reverse reactions, such as O2 photoreduction, must be addressed to prevent unnecessary decreases in photocatalytic activity. To overcome these challenges, a dual-chamber photocatalytic hydrogen production system was developed by a research group at RMIT, Australia[49]. This system comprises a photoanode and a photocathode for generating O2 and H2, respectively, and a PEM to transfer protons from the photoanode. The specialized structural design allows the system to float on water and maintain stability in moving seawater (Figure 2b). Furthermore, the precise energy band configuration establishes a Z-scheme charge transfer pathway, retaining charge carriers with strong redox abilities for chemical reactions while allowing those with weaker redox abilities to recombine and dissipate as heat or light (Figure 2c). Specifically, photogenerated holes at the photoanode oxidize water molecules to produce H+ and O2. The generated H+ then passes through the PEM and reacts with photoelectrons at the photocathode to form H2, achieving effective spatial separation of H2 and O2 in different chambers and reducing the risk of explosion. The dual-chamber elements can be modularly combined to form systems of desired size, similar to stacking building blocks (Figure 2d). By optimizing the pairing of hydrogen evolution and oxygen evolution catalysts, a hydrogen-to-oxygen ratio of 2:1 was ultimately achieved, indicating a reduction in side reactions.
Photocatalytic large-scale hydrogen production technology is currently at a critical stage of transitioning from laboratory research to industrial application. The estimated hydrogen production costs for single- and dual-bed particle suspension systems are US$ 1.6 and US$ 3.2 per kilogram, respectively, assuming STH efficiencies of 10% and 5% over a five-year operational lifetime. In contrast, photoelectrochemical systems exhibit higher costs of US$ 10.4 and US$ 4.0 per kilogram, assuming STH efficiencies of 10% and 15% and 10-year operational lifetime, respectively[50]. Suspended photocatalyst systems using simple plastic bag reactors demonstrate significantly lower costs compared with photoelectrochemical alternatives. Consequently, photocatalysis shows promise for achieving hydrogen production costs potentially with the US$ 2-4 per kilogram target range established by the U.S[8]. Department of energy under moderate STH efficiency and lifetime conditions. Beyond reasonable photolysis device design, another major challenge lies in the trade-off between economically affordable hydrogen and the high cost of panel reactors. This drives researchers to develop advanced photocatalysts and reactor designs to reduce overall costs. Achieving large-scale hydrogen production requires photocatalysts with the STH efficiency of at least 10% and the ability to maintain long-term stability for ten years. Simultaneously, this necessitates that the cost of photocatalytic panels does not exceed US$100 per square meter[51]. Table 1 summarizes the typical photocatalysts developed for large-scale hydrogen production, including apparent quantum efficiency, STH, performance, and stability.
| Photocatalysts | AQE (%) | STH (%) | Performance (mmol h-1) | Stability (h) | Ref. |
| Pt@g-C3N4 | - | 0.12 | 1.11 | 900 | [44] |
| Carbon nanodot-C3N4 | 16@420 nm | 2 | 8.4 | 4,800 | [52] |
| MoSe2 doping CH(NH2)2PbBr3-xIx | 33.96@530 nm | 2.47 | 1.18 | 60 | [53] |
| SrTaO2N | 0.34@420 ± 30 nm | 6.3 × 10-3 | 163 | 18 | [54] |
| Aza-fused microporous polymers/C2N | 4.3@600 nm | 0.73 | 22.5 | 32 | [55] |
| Al-doped SrTiO3 | 96@350-360 nm | 0.65 | 0.25 | 12.5 | [56] |
| Fe-g-C3N4 | 0.8@400-750 nm | - | 16.2 | - | [57] |
| InGaN/GaN | - | 9.2 | - | 10 | [58] |
AQE: apparent quantum efficiency; STH: solar-to-hydrogen.
4. Conclusion and Perspective
Over the past few decades, significant progress has been achieved in large-scale photocatalytic water splitting, particularly in catalyst and device design. Fundamental design principles for catalyst structure and physicochemical properties have been established to achieve high-efficiency photocatalysts at the laboratory scale, as well as to ensure the safe generation and separation of H2 and O2 mixtures during pilot-scale validation[59]. The use of artificial intelligence (AI) in designing high-efficiency photocatalysts and novel photocatalytic reactors helps reduce time lost to trial-and-error approaches, thereby improving the efficiency of photocatalyst and reactor development. Nevertheless, scaling up photocatalytic hydrogen production to the industrial level remains challenging, due to limitations in photocatalytic performance, reactor costs, and other factors. Continued advancements in both photocatalysts and their associated reactor systems are therefore essential. Future improvements should focus on the following areas:
(1) Catalytic performance and stability improvement for photocatalysts: Significant enhancements in the performance of photocatalytic materials are a prerequisite for the practical implementation of large-scale hydrogen production systems. Currently, the STH conversion efficiency of most photocatalytic systems for large-scale hydrogen production is below 2%, primarily because the photocatalysts absorb only ultraviolet light[8]. Improving the STH conversion efficiency to values above 1.7% remains highly challenging, motivating researchers to explore narrow-band semiconductors with bandgap energies below 2.1 eV to extend light absorption into the visible range. However, the low apparent quantum yield (AQY) under visible light remains a critical limitation for most visible-light-driven photocatalysts in water splitting, with reported AQY values seldom exceeding 10% even in the presence of sacrificial reagents[38]. Traditional photocatalytic hydrogen production typically relies on sacrificial agents, such as methanol or triethanolamine, to consume photogenerated holes and promote charge separation[60]. The oxidation of these sacrificial agents, however, is often non-selective, generating low-value byproducts and causing economic inefficiency. Transforming this process into selective oxidation to produce high-value chemicals can significantly enhance the economic viability of the overall system. Biomass photocatalytic refining offers a promising strategy in this regard. Biomass derivatives, such as glucose, xylose, and glycerol, can act as efficient sacrificial agents, while their selective oxidation products, including formic acid, furfural, and dihydroxyacetone, possess substantial market value[61]. Consequently, conventional hydrogen production systems could be upgraded into multi-purpose platforms, simultaneously generating hydrogen and high-value chemicals, thereby enhancing the economic feasibility of large-scale hydrogen production[62]. Regarding photocatalyst stability, deactivation and instability, primarily caused by photocorrosion and surface contamination, pose significant challenges for large-scale applications. These issues can be effectively mitigated through protective core-shell structures and periodic mild acid washing. Therefore, there is a compelling and urgent need for innovative strategies in the design, synthesis, and characterization of advanced photocatalytic systems, with a focus on improving stability and enabling the synergistic co-production of hydrogen and high-value chemicals.
(2) Safety and scalability enhancement for reactors: For the advancement of large-scale photocatalytic hydrogen production, operational safety and reactor scalability are of paramount importance. A key requirement for adapting to diverse terrain and operational conditions is modular scalability, which can be achieved through the assembly of standardized modules. This approach is critical for meeting varying operational requirements across different regions. Moreover, the reliance on pure water in existing photoreactor systems presents a major limitation for large-scale deployment. Therefore, designing reactors capable of direct photocatalytic seawater hydrolysis is essential. Photocatalytic seawater splitting, however, remains a formidable challenge because the diverse ionic constituents in seawater can induce catalyst deactivation through surface passivation and also pose significant risks to membrane-based separation technologies, leading to fouling and mechanical degradation. Ensuring gas separation safety is another critical concern, which can be addressed by employing AEMs to spatially separate gases into different chambers or by constructing filtration elements from hydrogen-permeable materials, such as commercial polyimide hollow fibers. In summary, overcoming the dual challenges of scalability and safety is essential for the practical implementation of large-scale photocatalytic hydrogen production technology.
(3) Cost considerations: The economic viability of photocatalytic water splitting technology presents a significant challenge, primarily determined by two cost factors: the research and development required for performance enhancement and the manufacturing expenses of photocatalytic platforms. The central economic challenge is that the value of the produced hydrogen must offset these costs. Given the current low market price of hydrogen, this can only be achieved if the technology simultaneously satisfies two stringent conditions: (i) achieving a STH conversion efficiency greater than 10%, and (ii) reducing the production cost of photocatalytic panels to under US$100 per square meter. To date, photoreactors developed for water splitting have demonstrated sufficient durability for academic research. However, a critical barrier to commercialization is that their associated costs remain significantly higher than the economically permissible limit. Therefore, a key research focus is the engineering of simpler reactor systems constructed from inexpensive and abundant materials. These systems must simultaneously provide operational stability and effective gas separation functionality. By simplifying reactor design or adopting a modular construction strategy, it is possible to achieve dual benefits: a substantial reduction in manufacturing costs and streamlined installation procedures, while also enabling flexible reaction devices of varying areas to meet diverse operational demands. This cost reduction is pivotal because it allows for a modest decrease in photocatalytic STH conversion efficiency without compromising the overall feasibility of the technology. Furthermore, integration with automation, data-driven analysis, and intelligent control enables AI to significantly reduce costs across the entire process, from fundamental research to large-scale application. These advancements not only accelerate progress in photocatalysis but also promote sustainable development in critical areas such as environmental protection and energy conversion. Examples include high-throughput screening to lower research and development costs, machine learning to optimize the design of effective catalysts such as single-atom catalysts, and intelligent control systems to minimize operational and maintenance expenses. Overall, progress in both performance enhancement and scalable, low-cost panel fabrication is essential for the commercial deployment of this technology.
(4) AI-assisted large-scale photocatalytic hydrogen production: has transformed the traditional design of photocatalysts, enabling a shift from trial-and-error methods to targeted, data-driven approaches. It allows rapid analysis of the complex interactions between material composition, structure, and photocatalytic activity, while accurately predicting critical parameters such as energy band structure, light absorption characteristics, and charge separation efficiency. Moreover, AI provides mechanistic insights into catalytic reactions and structure-property relationships, significantly accelerating research and development cycles and reducing associated costs[63]. In photocatalytic reactor design, AI employs intelligent algorithms to optimize reactor structures, optical pathways, and fluid dynamics by integrating with computational fluid dynamics. This integration can precisely simulate multi-field coupling processes, including light energy distribution, mass transfer, and reaction kinetics, thereby achieving coordinated optimization of reactor configurations[64]. Additionally, AI enables real-time regulation of operational parameters such as light intensity, temperature, and reactant concentration through digital control systems. These capabilities enhance system operational stability and STH conversion efficiency, advancing the development of photocatalytic hydrogen production reactors toward high efficiency, intelligence, and scalability.
Authors contribution
Wang XS: Data analysis, investigation, validation, writing-original draft, writing-review & editing.
Yuan ZY: Conceptualization, project administration, supervision, validation, writing-review & editing.
All authors have given approval to the final version of the manuscript.
Conflicts of interest
Zhong-Yong Yuan is the Editor-in-Chief of Smart Materials and Devices. No other conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (22179065).
Copyright
© The Author(s) 2025.
References
-
1. Ren JT, Chen L, Wang HY, Yuan ZY High-entropy alloys in electrocatalysis: from fundamentals to applications. Chem Soc Rev. 2023;52(23):8319-8373.[DOI]
-
2. Wang HY, Yuan ZY. Hydrazine-assisted water electrolysis system: performance enhancement and application expansion. Mater Horiz. 2025;12:5123-5148.[DOI]
-
3. Ren JT, Chen L, Wang HY, Tian WW, Yuan ZY. Water electrolysis for hydrogen production: from hybrid systems to self-powered/catalyzed devices. Energy Environ Sci. 2024;17(1):49-113.[DOI]
-
4. Simon T, Bouchonville N, Berr MJ, Vaneski A, Adrović A, Volbers D, et al. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat Mater. 2014;13(11):1013-1018.[DOI]
-
5. Gao M, Tian F, Zhang X, Chen Z, Yang W, Yu Y. Improved plasmonic hot-electron capture in Au nanoparticle/polymeric carbon nitride by Pt single atoms for broad-spectrum photocatalytic H2 evolution. Nano Micro Lett. 2023;15:129.[DOI]
-
6. Kim JH, Hansora D, Sharma P, Jang JW, Lee JS. Toward practical solar hydrogen production - an artificial photosynthetic leaf-to-farm challenge. Chem Soc Rev. 2019;48(7):1908-1971.[DOI]
-
7. Jia J, Seitz LC, Benck JD, Huo Y, Chen Y, Ng JW, et al. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat Commun. 2016;7(1):13237.[DOI]
-
8. Hisatomi T, Domen K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat Catal. 2019;2(5):387-399.[DOI]
-
9. Chen L, Ren JT, Yuan ZY. Enabling internal electric fields to enhance energy and environmental catalysis. Adv Energy Mater. 2023;13(11):2203720.[DOI]
-
10. Wang C, Zhao Y, Cheng C, Li Q, Guo C, Hu Y. S-scheme heterojunction photocatalysts: Mechanism, challenges and opportunities. Coord Chem Rev. 2024;521:216177.[DOI]
-
11. Low J, Yu J, Jaroniec M, Wageh S, Al‐Ghamdi AA. Heterojunction photocatalysts. Adv Mater. 2017;29(20):1601694.[DOI]
-
12. Li R, Li C. Scalable solar water splitting using particulate photocatalysts. Curr Opin Green Sustain Chem. 2022;33:100577.[DOI]
-
13. Tu S, Zhang Y, Reshak AH, Auluck S, Ye L, Han X, et al. Ferroelectric polarization promoted bulk charge separation for highly efficient CO2 photoreduction of SrBi4Ti4O15. Nano Energy. 2019;56:840-850.[DOI]
-
14. Yu S, Li J, Zhang Y, Li M, Dong F, Zhang T, et al. Local spatial charge separation and proton activation induced by surface hydroxylation promoting photocatalytic hydrogen evolution of polymeric carbon nitride. Nano Energy. 2018;50:383-392.[DOI]
-
15. Hao L, Kang L, Huang H, Ye L, Han K, Yang S, et al. Surface-halogenation-induced atomic-site activation and local charge separation for superb CO2 photoreduction. Adv Mater. 2019;31(25):1900546.[DOI]
-
16. Chen F, Huang H, Guo L, Zhang Y, Ma T. The role of polarization in photocatalysis. Angew Chem Int Ed. 2019;58(30):10061-10073.[DOI]
-
17. Zhang L, Zhang J, Yu J, García H. Charge-transfer dynamics in S-scheme photocatalyst. Nat Rev Chem. 2025;9:328-342.[DOI]
-
18. Ba G, Hu H, Bi F, Yu J, Liu E, Ye J, et al. Engineering nitrogen vacancies and cyano groups into C3N4 nanosheets for highly efficient photocatalytic H2O2 production. Appl Catal B Environ Energy. 2025;361:124645.[DOI]
-
19. Kwon NH, Kim SJ, Gu TH, Lee JM, Kim MH, Paik D, et al. Surface optimization of noble-metal-free conductive [Mn1/4Co1/2Ni1/4]O2 nanosheets for boosting their efficacy as hybridization matrices. Adv Sci. 2024;11(44):2408948.[DOI]
-
20. Khan MA, Mutahir S, Shaheen I, Qunhui Y, Bououdina M, Humayun M. Recent advances over the doped g-C3N4 in photocatalysis: a review. Coord Chem Rev. 2025;522:216227.[DOI]
-
21. Jiang L, Yuan X, Pan Y, Liang J, Zeng G, Wu Z, et al. Doping of graphitic carbon nitride for photocatalysis: a review. Appl Catal B Environ. 2017;217:388-406.[DOI]
-
22. Li J, Yuan H, Zhang W, Jin B, Feng Q, Huang J, et al. Advances in Z-scheme semiconductor photocatalysts for the photoelectrochemical applications: a review. Carbon Energy. 2022;4(3):294-331.[DOI]
-
23. He W, Liu L, Ma T, Han H, Zhu J, Liu Y, et al. Controllable morphology CoFe2O4/g-C3N4 p-n heterojunction photocatalysts with built-in electric field enhance photocatalytic performance. Appl Catal B Environ. 2022;306:121107.[DOI]
-
24. Zhang J, Liu J, Meng Z, Jana S, Wang L, Zhu B. Electron transfer dynamics in Schottky junction photocatalyst during electron donor-assisted hydrogen production. J Mater Sci Technol. 2023;159:1-9.[DOI]
-
25. Meng K, Zhang J, Cheng B, Ren X, Xia Z, Xu F, et al. Plasmonic near-infrared-response S-scheme ZnO/CuInS2 photocatalyst for H2O2 production coupled with glycerin oxidation. Adv Mater. 2024;36(32):2406460.[DOI]
-
26. Zu D, Wei H, Lin Z, Bai X, Ivan MN, Tsang YH, et al. The role of point defects in heterojunction photocatalysts: perspectives and outlooks. Adv Funct Mater. 2024;34(48):2408213.[DOI]
-
27. Li Y, Zhang J, Chen Q, Xia X, Chen M. Emerging of heterostructure materials in energy storage: a review. Adv Mater. 2021;33(27):2100855.[DOI]
-
28. Ye MY, Zhao ZH, Hu ZF, Liu LQ, Ji HM, Shen ZR, et al. 0D/2D heterojunctions of vanadate quantum dots/graphitic carbon nitride nanosheets for enhanced visible-light-driven photocatalysis. Angew Chem Int Ed. 2017;56(29):8407-8411.[DOI]
-
29. Shi L, Xin T, Peng C, Wu Z, Liu S, Yu K, et al. UiO series of MOFs and their composites for photocatalytic CO2 reduction: a review. Smart Mater Devices. 2025;1:202506.[DOI]
-
30. Liu X, Zhang Y, Sun P, He F, Wu Y, Wang S, et al. Asymmetric coordination in cobalt single-atom catalysts enables fast charge dynamics and hierarchical active sites for two-stage kinetics in photodegradation of organic pollutants. Angew Chem. 2025;137(28):e202507028.[DOI]
-
31. Liu Y, Deng A, Yin Y, Lin J, Li Q, Sun Y, et al. Modulation of catalyst microenvironments in ZnIn2S4/g-C3N4 S-scheme heterojunction for ratio-tunable syngas production from CO2 photoreduction. Appl Catal B Environ Energy. 2025;362:124724.[DOI]
-
32. Li C, Lu H, Ding G, Ma T, Liu S, Zhang L, et al. Interfacial coordination bonds accelerate charge separation for unprecedented hydrogen evolution over S-scheme heterojunction. Chin J Catal. 2024;65:174-184.[DOI]
-
33. Guo X, Fan L, Liu J, Wen B, Li Y, Jin Z. Built-in electric field and oxygen vacancy defects in ZnCdS/Ov-NiCo2O4 schottky heterojunction to achieve efficient photocatalytic hydrogen evolution. Appl Catal B Environ Energy. 2025;378:125586.[DOI]
-
34. Rao VN, Pitchaimuthu S, Ravi P, Sathish M, Han H, Venkatakrishnan SM. Retorting photocorrosion and enhanced charge carrier separation at CdSe nanocapsules by chemically synthesized TiO2 shell for photocatalytic hydrogen fuel generation. ChemCatChem. 2020;12(11):3139-3152.[DOI]
-
35. Mani P, Shenoy S, Sagayaraj PJ, Agamendran N, Son S, Bernaurdshaw N, et al. Scaling up of photocatalytic systems for large-scale hydrogen generation. Appl Phys Rev. 2025;12(1):11303.[DOI]
-
36. Lyons RJ, Sprick RS. Sprick, Processing polymer photocatalysts for photocatalytic hydrogen evolution. Mater Horiz. 2024;11(16):3764-3791.[DOI]
-
37. Li Y, Yuan Z, Wang J, Xu Q. Laser-induced damage characteristics in fused silica surface due to mechanical and chemical defects during manufacturing processes. Opt Laser Technol. 2017;91:149-158.[DOI]
-
38. Li L, Tang H, Chen Y, Yang R, Tian D, Chen Z. Effect of lactic acid on the photoelectrocatalytic water splitting of hematite prepared by hydrothermal method. Electron Mater Lett. 2020;(16):481-490.[DOI]
-
39. Zhang W, Ye C, Liu Q. Development of anti-reflective coatings with photocatalytic and hydrophobic self-cleaning property for solar cells. Surf Interfaces. 2025;65:106454.[DOI]
-
40. Li F, Liu G, Liu F, Yang S. A review of self-cleaning photocatalytic surface: effect of surface characteristics on photocatalytic activity for NO. Environ Pollut. 2023;327:121580.[DOI]
-
41. Mendoza-Diaz MI, Lecestre A, Salvagnac L, Bounor B, Pech D, Djafari-Rouhani M, et al. High surface area TiO2 photocatalyst for H2 production through silicon micromachining. Appl Surf Sci. 2022;588:152919.[DOI]
-
42. Meng F, Hao T, Tian W, Zhao J, Wang S, Zhang H. Photocatalytic aqueous environmental remediation via two-dimensional carbon nitride nanosheets. Surf Interfaces. 2024;44:103717.[DOI]
-
43. Maurya J, Gemechu E, Kumar A. The development of techno-economic assessment models for hydrogen production via photocatalytic water splitting. Energy Convers Manag. 2023;279:116750.[DOI]
-
44. Schröder M, Kailasam K, Borgmeyer J, Neumann M, Thomas A, Schomäcker R, et al. Hydrogen evolution reaction in a large-scale reactor using a carbon nitride photocatalyst under natural sunlight irradiation. Energy Technol. 2015;3(10):1014-1017.[DOI]
-
45. Nishiyama H, Yamada T, Nakabayashi M, Maehara Y, Yamaguchi M, Kuromiya Y, et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature. 2021;598(7880):304-307.[DOI]
-
46. Lee WH, Lee CW, Cha GD, Lee BH, Jeong JH, Park H, et al. Floatable photocatalytic hydrogel nanocomposites for large-scale solar hydrogen production. Nat Nanotechnol. 2023;18(7):754-762.[DOI]
-
47. Tachibana Y, Vayssieres L, Durrant JR. Artificial photosynthesis for solar water-splitting. Nat Photonics. 2012;6(8):511-518.[DOI]
-
48. Maeda K, Domen K. Photocatalytic water splitting: recent progress and future challenges. J Phys Chem Lett. 2010;1(18):2655-2661.[DOI]
-
49. Ma T. Clean energy storage via hydrogen and hydrocarbon: From photocatalytic generation to device implementation [Internet]. Science Exploration Press; 2025 Jul 17. Available from: https://sciexplor.com/webinars/smd/14
-
50. Pinaud BA, Benck JD, Seitz LC, Forman AJ, Chen Z, Deutsch TG, et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ Sci. 2013;6(7):1983-2002.[DOI]
-
51. Hisatomi T, Yamada T, Nishiyama H, Takata T, Domen K. Materials and systems for large-scale photocatalytic water splitting. Nat Rev Mater. 2025;14:1-4.[DOI]
-
52. Liu J, Liu Y, Liu N, Han Y, Zhang X, Huang H, et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science. 2015;347(6225):970-974.[DOI]
-
53. Fu H, Wu Y, Guo Y, Sakurai T, Zhang Q, Liu Y, et al. A scalable solar-driven photocatalytic system for separated H2 and O2 production from water. Nat Commun. 2025;16(1):990.[DOI]
-
54. Chen K, Xiao J, Vequizo JJ, Hisatomi T, Ma Y, Nakabayashi M, et al. Overall water splitting by a SrTaO2 N-based photocatalyst decorated with an Ir-promoted Ru-based cocatalyst. J Am Chem Soc. 2023;145(7):3839-3843.[DOI]
-
55. Wang L, Zheng X, Chen L, Xiong Y, Xu H. Van der waals heterostructures comprised of ultrathin polymer nanosheets for efficient Z-scheme overall water splitting. Angew Chem Int Ed. 2018;57(13):3454-3458.[DOI]
-
56. Takata T, Jiang J, Sakata Y, Nakabayashi M, Shibata N, Nandal V, et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature. 2020;581(7809):411-414.[DOI]
-
57. Gao LF, Wen T, Xu JY, Zhai XP, Zhao M, Hu GW, et al. Iron-doped carbon nitride-type polymers as homogeneous organocatalysts for visible light-driven hydrogen evolution. ACS Appl Mater Interfaces. 2026;8(1):617-624.[DOI]
-
58. Zhou P, Navid IA, Ma Y, Xiao Y, Wang P, Ye Z, et al. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature. 2023;613:66-70.[DOI]
-
59. Li J, Li Z, Sun Q, Wang Y, Li Y, Peng YK, et al. Recent advances in the large-scale production of photo/electrocatalysts for energy conversion and beyond. Adv Energy Mater. 2024;14(45):2402441.[DOI]
-
60. Kumaravel V, Imam MD, Badreldin A, Chava RK, Do JY, Kang M, et al. Photocatalytic hydrogen production: role of sacrificial reagents on the activity of oxide, carbon, and sulfide catalysts. Catalysts. 2019;9(3):276.[DOI]
-
61. Li H, Zhao H, Dong Y, Zhu Y, Li J. Highly Selective Photocatalytic Oxidation Biomass Valorization Over Nb2O5/g‐C3N4 Heterojunction. Adv Energy Sustain Res. 2022;3(12):2200116.[DOI]
-
62. Llatance-Guevara L, Flores NE, Barrionuevo GO, Mullo Casillas JL. Waste biomass selective and sustainable photooxidation to high-added-value products: a review. Catalysts. 2022;12(10):1091.[DOI]
-
63. Gonuguntla S, Jaksani B, Jamma A, Vennapoosa CS, Chatterjee D, Pal U. Design principle of anti-corrosive photocatalyst for large-scale hydrogen production. WIREs Energy Environ. 2024;13(4):e530.[DOI]
-
64. Arsad AZ, Hannan MA, Ong HC, Ker PJ, Wong RT, Begum RA, et al. Artificial intelligence in hydrogen energy transitions: a comprehensive survey and future directions. Renew Sustain Energy Rev. 2025;224:116121.[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite