Jianxin Geng, Tianjin Key Laboratory of Advanced Fibers and Energy Storage, School of Material Science and Engineering, Tiangong University, Tianjin 300387, China. E-mail: jianxingeng@tiangong.edu.cn
Rechargeable zinc-air batteries (R-ZABs) have garnered significant attention as promising energy storage devices, attributed to their low cost, inherent safety, and good sustainability[1]. Despite these advantages, the practical application of R-ZABs is hindered by the sluggish kinetics displayed by the oxygen reduction and evolution reactions (i.e., ORR and OER)[2]. So far, efforts have focused on improving the discharge performance of R-ZABs through the use of high-activity ORR catalysts, such as metal-nitrogen-carbon (M-N-C), single/dual-atom catalysts (SACs/DACs), and high-entropy alloys[3-5]. Unfortunately, the sluggish OER (1.23 V vs. RHE) during charging has been largely overlooked. Its high thermodynamic equilibrium potential not only escalates the energy input required for charging (often exceeding 1.9 V) but also leads to catalyst degradation, thereby shortening the useful lifespan of R-ZABs[6].
To address this challenge, the OER in traditional R-ZABs can be replaced with favorable oxidation reactions of small molecules, such as urea, methanol, and ethanol[7-9]. Among these alternatives, the urea oxidation reaction (UOR, CO(NH2)2 + 6OH- → N2 + CO2 + 5H2O + 6e-, 0.37 V vs. RHE) stands out as an ideal anode half-reaction for clean energy conversion and storage devices (Figure 1a,b)[10-12]. The unique advantages of the UOR over other small-molecule oxidations are as follows: (i) compared to unstable and highly toxic hydrazine, urea is more stable in alkaline solutions and less toxic; (ii) urea is a common pollutant in wastewater. Utilizing the UOR not only reduces the charging potential but also purifies wastewater, a benefit that alcohol oxidation cannot provide. Nevertheless, the UOR suffers from torpid kinetics, involving multiple electron transfers, complex intermediate conversions, and CO poisoning[13]. Therefore, it is highly crucial to develop high-activity and stable UOR catalysts.
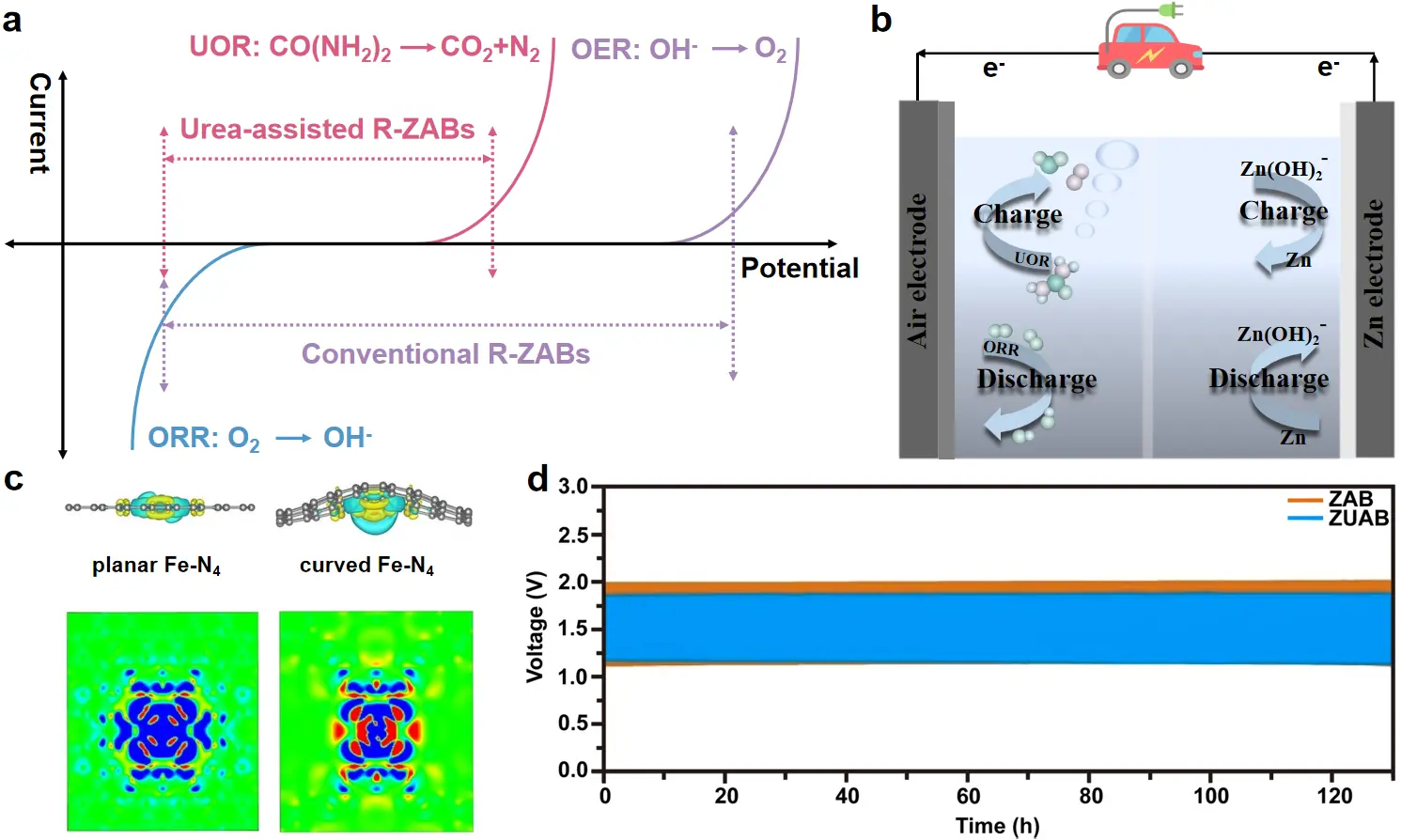
Figure 1. (a) Comparison of the potential gap between the anodic and cathodic reactions in conventional and urea-assisted R-ZABs; (b) Schematic diagram of urea-assisted R-ZABs. R-ZABs: Rechargeable zinc-air batteries.
Current research on UOR catalysts primarily focuses on three material categories: (i) Bulk Ni/Co-based catalysts, which attract extensive attention for their readily tunable 3d orbital electron configurations and high intrinsic activity[14-16]. A self-supported Ag-CoOOH@NiOOH heterojunction, constructed by coupling an Ag-CoOOH nanosheet with a compact NiOOH layer, achieves high-energy interfacial structures[16]. Such strong interactions enhance catalytic activity and selectivity for UOR, ensuring good stability. (ii) SACs, which maximize atom utilization efficiency and provide unique electronic properties for enhanced catalytic performance. For instance, Ni SAsNC exhibit near 100% atom efficiency and dual ORR/UOR functionality for urea-assisted R-ZABs[12]. (iii) DACs, which optimize the adsorption and activation of urea molecules, thereby improving catalytic activity and selectivity. For example, Co-Ni DACs synergistically combine the properties of two different metals to show promise in the UOR process[17]. Despite this progress, current research is still in the development stage regarding the regulation of the microscopic geometric structure of catalyst active sites for efficient UOR. Compared to traditional methods (e.g., doping, alloying, and constructing heterojunctions), curvature-induced intrinsic microstrain enables more precise and tunable regulation of metal center electronic structures, offering fine-tuning of the coordination environment around the active metal centers and effective modulation of their electronic properties[18,19].
In a recent study, Kang and co-workers developed Fe SAs anchored on a highly curved NC dodecahedron with a concave morphology (Fe SA/NhcC)[20], which served as a superior bifunctional catalyst for both the ORR and UOR in urea-assisted R-ZABs. Curvature engineering has been proven to enable precise geometric and electronic control, directly manipulating bond lengths and coordination geometries of metal centers, which is crucial for optimizing catalytic activity. Additionally, the three-dimensional curvature enhances mass transport and increases accessible active site densities, significantly boosting catalytic efficiency.
By comparing SEM and TEM images, the morphology of Fe SA/NC differs significantly from that of Fe SA/NhcC, revealing the presence of curved surfaces (Figure 2a,b,c,d). This is supported by extended X-ray absorption fine structure (EXAFS) data. The average Fe-N bond length in Fe SA/NhcC was slightly shorter than that of Fe SA/NC (i.e., 1.92 vs. 2.01 Å, Figure 2e), indicating the formation of a local strain effect induced by the curved carbon nanostructure. Such high surface curvature of the carbon support facilitates fine-tuning of the spatial charge redistribution at Fe-N4 sites (Figure 2f). Moreover, the curved Fe-N4 site with moderate curvature was proven to show superior intrinsic ORR/UOR activity relative to the zero-strain planar Fe-N4 site. The resultant urea-assisted R-ZAB prepared with Fe SA/NhcC exhibited an ultra-low charge-discharge voltage difference of 0.686 V, significantly lower than that of conventional R-ZABs. It also exhibits long-term cycling stability for 130 h at 20 mA cm-2.
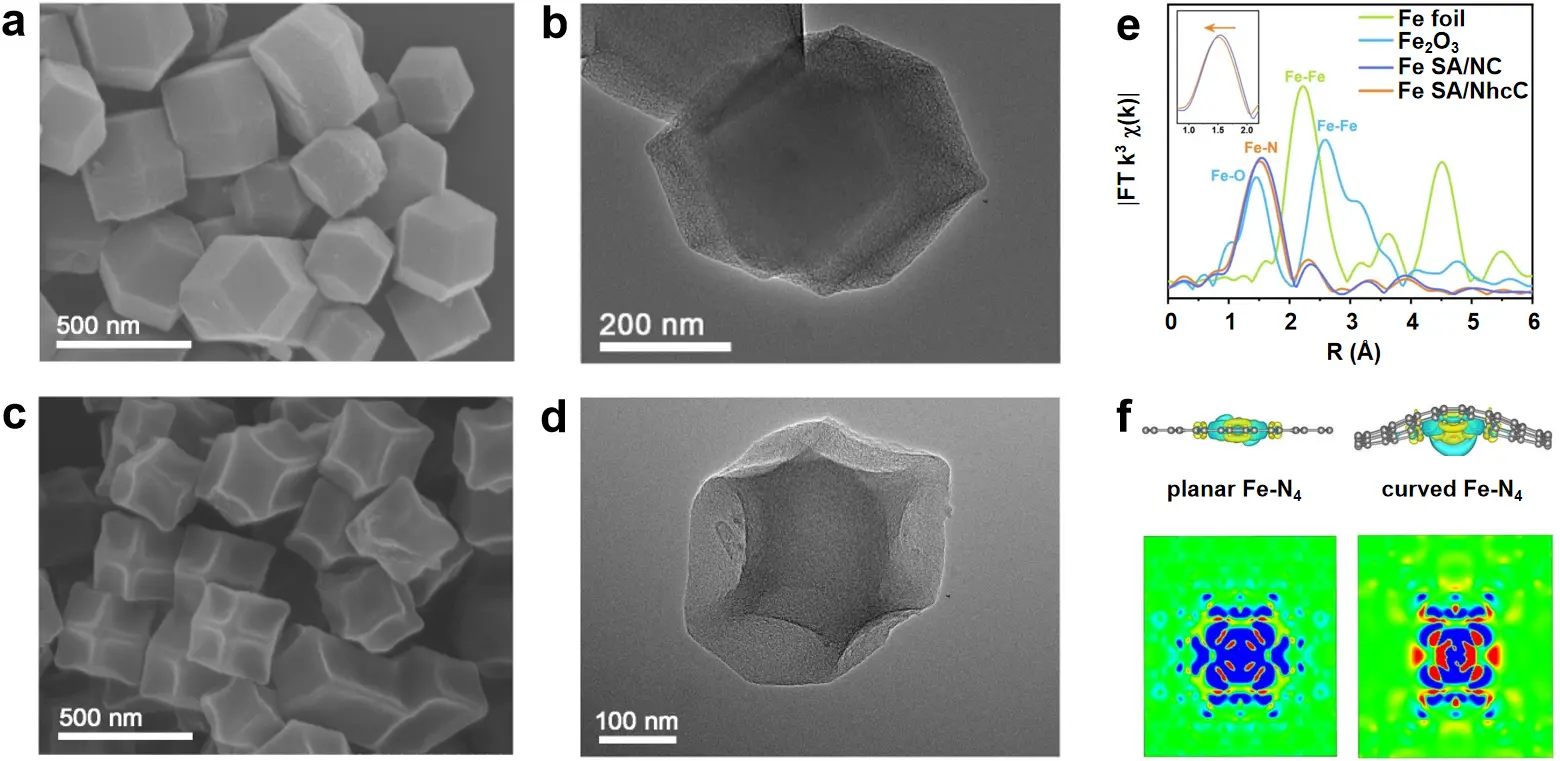
Figure 2. (a-d) SEM and TEM images of (a, b) Fe SA/NC and (c, d) Fe SA/NhcC; (e) FT spectra as obtained from the k3-weighted Fe K-edge EXAFS data recorded for Fe SA/NC, Fe SA/NhcC, Fe foil and Fe2O3; (f) Charge density difference (up) and 2D contour plot (down) for planar and curved Fe-N-C. Republished with permission from[20]. SEM: scanning electron microscopy; TEM: transmission electron microscopy; SA: single atom; NhcC: highly curved N-doped carbon dodecahedron with concave morphology; FT: fourier Transform; EXAFS: extended X-ray Absorption fine structure.
In summary, this work provides valuable insights into enhancing the catalytic activity of SACs through modulating the surface curvature and the geometric configuration. Importantly, the as-constructed urea-assisted R-ZABs catalyzed by Fe SA/NhcC offer a new approach for concurrent fast-charging kinetics and contaminant urea removal. Looking ahead, several critical aspects warrant further exploration. (i) The scalability of SAC synthesis is essential for practical applications. Current synthesis methods often face challenges in maintaining the high dispersion and stability of SAs when scaled up. To address this, potential strategies such as template-free synthesis or continuous flow reactors can be employed to achieve scaled-up production of SACs while maintaining single-atom dispersion. (ii) Actual industrial wastewater contains a myriad of impurities that may interfere with the UOR process and affect catalyst stability. Future research should focus on enhancing the robustness of catalysts to withstand such complex environments, potentially through surface modification (e.g., functionalization with hydrophobic groups, grafting of polymeric layers) or the development of protective coatings (e.g., carbon encapsulation, atomic layer deposition). (iii) The integration of these catalysts into large-scale battery devices poses significant engineering challenges. Efficient electrode design, optimized mass transport, and system integration are crucial for realizing the full potential of urea-assisted R-ZABs in real-world scenarios. Key engineering priorities include the development of scalable electrode architectures and the optimization of electrolyte flow to ensure efficient mass transport.
Acknowledgements
AI-assisted tools were used for language editing only. The authors are responsible for the accuracy and scientific content of the article.
Authors contribution
Gong J: Data analysis, investigation, validation, writing-original draft, writing-review & editing. Meng T: Data analysis, investigation.
Lv XW, Geng J: Conceptualization, project administration, supervision, validation, writing-review & editing.
All authors have given approval to the final version of the manuscript.
Conflicts of interest
Jianxin Geng is an Editorial Board Member of Smart Materials and Devices. The other authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (52273215, 52311540152), and the Natural Science Foundation of Tianjin (25JCYBJC00770).
Copyright
© The Author(s) 2026.
References
-
1. Li YR, Xu JQ, Lan F, Wang Y, Jiang H, Zhu P, et al. Atomic-level tin regulation for high-performance zinc-air batteries. J Am Chem Soc. 2025;147(6):4833-4843.[DOI]
-
2. Zou XH, Lu Q, Wu LZ, Zhang K, Tang MC, Xie HJ, et al. I3--mediated oxygen evolution activities to boost rechargeable zinc-air battery performance with low charging voltage and long cycling life. Angew Chem Int Ed. 2025;64(4):e202416235.[DOI]
-
3. Yan Y, Wen B, Liu M, Lei H, Yang J, He S, et al. Orienting electron fillings in d orbitals of cobalt single atoms for effective zinc-air battery at a subzero temperature. Adv Funct Mater. 2024;34(30):2316100.[DOI]
-
4. Wu W, Wang Z, Zhu Y, Jiang H, Chen X, Chen S, et al. Dual-atomic-site engineering of Pt-based intermetallic catalysts for highly efficient and durable oxygen reduction. Adv Funct Mater. 2025;17:e14892.[DOI]
-
5. Chen T, Zhang X, Wang H, Yuan C, Zuo Y, Gao C, et al. Antisite defect unleashes catalytic potential in high-entropy intermetallics for oxygen reduction reaction. Nat Commun. 2025;16(1):3308.[DOI]
-
6. Qiao J, You Y, Kong L, Feng W, Zhang H, Huang H, et al. Precisely constructing orbital-coupled Fe-Co dual-atom sites for high-energy-efficiency Zn-air/iodide hybrid batteries. Adv Mater. 2024;36(32):2405533.[DOI]
-
7. Zhang X, Liu G, Zhao C, Wang G, Zhang Y, Zhang H, et al. Highly efficient electrocatalytic oxidation of urea on a Mn-incorporated Ni(OH)2/carbon fiber cloth for energy-saving rechargeable Zn-air batteries. Chem Commun. 2017;53(77):10711-10714.[DOI]
-
8. Gao D, Chen J, Zhang Y, Li Y, Zhu L, Lv Y, et al. Oxygen-bridged Ga-O-PtPd triple sites boost methanol-assisted rechargeable Zn-air batteries through suppressing coads generation. Adv Energy Mater. 2025;15(25):2500421.[DOI]
-
9. Li Z, Ning S, Jin Y, Wang N, Sun S, Meng H. Zinc-alcohol-air batteries with ultra-narrow cyclic voltage windows. Energy Environ Sci. 2025;18(2):1002-1010.[DOI]
-
10. Tian WW, Ren JT, Wang HY, Wang L, Yuan ZY. Bioinspired binary-site catalysts for novel urea-assisted Zn-air battery: A transfer station between renewable energy and hydrogen. App Catal B: Environ. 2024;354:124115.[DOI]
-
11. Li H, Liu Y, Huang L, Xin J, Zhang T, Liu P, et al. 2D Metal-organic framework derived Co/CoSe2 heterojunctions with interfacial electron redistribution as bifunctional electrocatalysts for urea-assisted rechargeable Zn-air batteries. J Mater Chem A. 2023;11(10):5179-5187.[DOI]
-
12. Jiang H, Xia J, Jiao L, Meng X, Wang P, Lee CS, et al. Ni single atoms anchored on N-doped carbon nanosheets as bifunctional electrocatalysts for Urea-assisted rechargeable Zn-air batteries. App Catal B: Environ. 2022;310:121352.[DOI]
-
13. Li T, Zheng Z, Chen Z, Zhang M, Liu Z, Chen H, et al. Ultrafast synthesis of an efficient urea oxidation electrocatalyst for urea-assisted fast-charging Zn–air batteries and water splitting. Energy Environ Sci. 2025;18(10):4996-5008.[DOI]
-
14. Jian J, Qiao Y, Chen F, Liu C, Liu W, Li Z, et al. Local charge density regulation of NiP2 nanosheets via Ru doping for electrocatalytic urea oxidation. App Cata. B: Environ. 2025;374:125390.[DOI]
-
15. Wang J, Sun M, Zhang X, Liu J, He J, Ge W, et al. pH-dependent urea electrooxidation: From mechanism to catalysts and applications. Adv Funct Mater. 2025;14:e15043.[DOI]
-
16. Wu M, Xu Y, Luo J, Yang S, Zhang G, Du L, et al. A rechargeable urea-assisted Zn-air battery with high energy efficiency and fast-charging enabled by engineering high-energy interfacial structures. Angew Chem Int Ed. 2024;63(49):e202410845.[DOI]
-
17. Zheng X, Yang J, Li P, Jiang Z, Zhu P, Wang Q, et al. Dual-atom support boosts nickel‐catalyzed urea electrooxidation. Angew Chem Int Ed. 2022;135(22):e202217449.[DOI]
-
18. Chen L, Ren JT, Li M, Giorgetti M, Yuan ZY. Enhancing aerobic oxidative desulfurization: Curvature-tailored local environments for efficient two-phase reactant delivery. App Catal B: Environ. 2025;379:125726.[DOI]
-
19. Kong QH, Lv XW, Weng CC, Ren JT, Tian WW, Yuan ZY. Curving engineering of hollow concave-shaped rhombic dodecahedrons of N‑doped carbon encapsulated with Fe-doped Co/Co3O4 nanoparticles for an efficient oxygen reduction reaction and Zn−air batteries. ACS Sustainable Chem Eng. 2022;10(34):11441-11450.[DOI]
-
20. Wang Q, Lyu L, Hu X, Fan W, Shang C, Huang Q, et al. Tailoring the surface curvature of the supporting carbon to tune the d-band center of Fe-N-C single-atom catalysts for zinc-urea-air batteries. Angew Chem Int Ed. 2025;64(15):e202422920.[DOI]
Copyright
© The Author(s) 2026. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite




