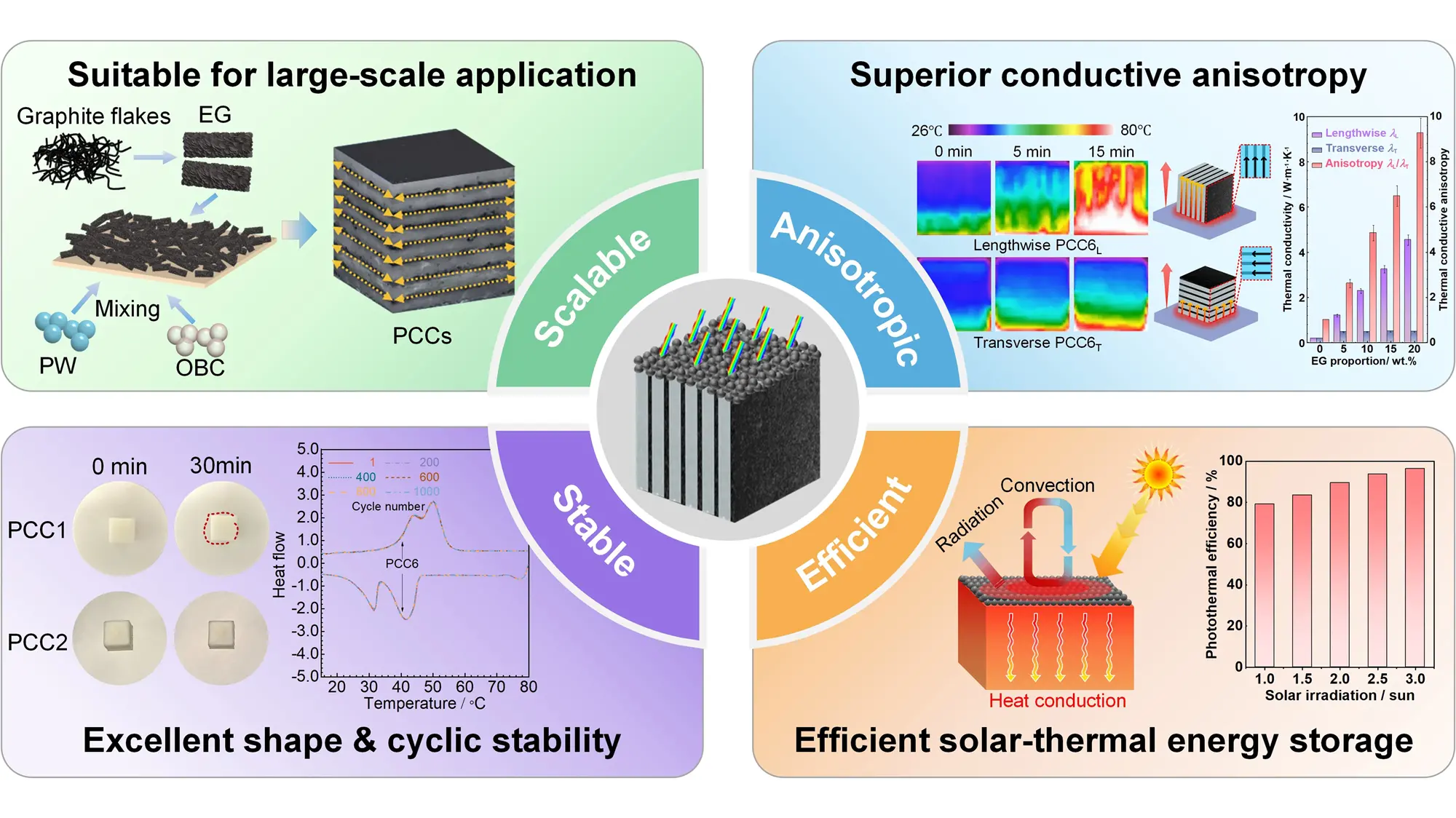
Abstract
Phase change materials possess significant potential for solar-thermal energy storage yet face critical limitations, including structural instability, inherently poor heat conductivity, and inadequate solar absorption, thereby constraining their practical applications. To address these challenges, we developed a laminated phase change composite (PCC) via pressure-assisted lamination of paraffin wax-olefin block copolymer (PW-OBC) with expanded graphite (EG) sheets. Experiments indicate that the OBC in the well-mixed PW-OBC sheet forms a three-dimensional network that encases the PW, enabling excellent leakage resistance, thermal/cyclic durability, and shape stability. The parallel EG sheets establish directional and continuous heat transport channels, resulting in 4.54 W·m-1·K-1 lengthwise heat conductivity versus a transverse value of 0.49 W·m-1·K-1, with an excellent thermal conductive anisotropy of 9.27. Coating the PCC surface with carbon black enhances its solar irradiation absorption, yielding a solar absorptivity of 0.98. Benefiting from the synergy of anisotropic heat conduction and enhanced solar absorption, the PCC can attain 79.2%-96.5% solar-thermal efficiency within 1-3 suns irradiance, enabling effective solar energy capture and storage. These results provide a viable approach for producing high-performance, anisotropically conductive PCCs for efficient low- to medium-temperature solar-thermal applications.
Graphical Abstract
Keywords
1. Introduction
Achieving net-zero global emissions of carbon dioxide while reducing emissions of other greenhouse gases is generally regarded as a promising way to curb global warming[1]. However, extensive fossil fuel utilization has been emitting massive greenhouse gases, bringing significant challenges to this goal. Consequently, transitioning to sustainable renewable energy has become extremely urgent. However, large-scale deployment of renewable energy still faces constraints due to the intermittency and variability of most renewable energy resources[2]. To overcome these constraints, thermal energy storage (TES), with its large capacity, high efficiency, and stability, provides a promising solution[3]. TES has already been widely applied in many renewable energy applications, such as solar thermal power and industrial process systems[4].
Recently, latent TES has attracted increasing attention for solar-thermal energy storage (STES) due to its stable phase change temperature and high energy storage density[5]. However, most phase change materials (PCMs) face limitations such as poor shape stability, low cycling durability, poor thermal conductivity[6], and insufficient solar absorption[7]. These issues result in leakage, slow heat transfer, and low solar-thermal efficiency during operation, severely restricting practical STES applications[8,9]. To overcome these challenges, phase change composites (PCCs) have been developed by modifying PCMs or adding functional fillers[10].
Enhancing the shape stability of PCCs can usually be achieved through either PCM confinement within supporting materials or inter-PCM polymerization. These strategies can be mainly divided into three approaches: hybrid confinement, encapsulation, and polymerization[11].
Hybrid confinement can restrict PCMs within the internal pores of porous media. For example, Baniasadi et al.[12] developed a PCC based on cellulose-based foams. The inherent high porosity of the foams significantly enhances the PCC’s shape stability, preventing any leakage even after being heating at 80 °C for 2 h. Wu et al.[13] mixed liquid paraffin wax-olefin block copolymer (PW-OBC) with expanded graphite (EG) through pressure-induced melt impregnation, where the cross-linked OBC matrix effectively reduces the PW leakage.
PCM encapsulation enhances the shape stability through encapsulating PCMs within a microscale coating or shell. For instance, Zhu et al.[14] fabricated shells through interfacial hydrolysis and polycondensation of Methyltrimethoxysilane and Tetraethyl orthosilicate, coupled with graphene self-assembly, to encapsulate n-octadecane, obtaining a PCC with satisfactory stability. Luo et al.[15] first grafted maleic acid ditetradecyl ester (MATD) onto graphene (GN) through the Diels-Alder reaction, obtaining a MATD-grafted GN (GM). Then, titanium dioxide (TiO2) was in situ self-assembled with GM, achieving relatively stable TiO2@GM composites that show a low leakage rate of 0.21%.
PCM polymerization enhances the shape stability of PCC through molecular bond reconstruction. For example, Sun et al.[16] fabricated a PCC by using dicumyl peroxide as a cross-linking agent. The internally cross-linked styrene-ethylene-butylene-styrene (SEBS) network enables the PCC to maintain structural integrity even above the melting point of PW, effectively preventing PW leakage. Yao et al.[17] developed a PCC with a dual 3D cross-linked network of olefin block copolymers and SEBS via a chemical cross-linking method. The PCC exhibits excellent shape stability, achieving a low leakage rate in water-air combined leakage tests (< 0.3% after 2 cycles).
To sum up, these approaches can effectively enhance the shape stability of PCCs but offer limited improvement in their solar-thermal performance. Low thermal conductivity and solar absorptance remain critical barriers to developing PCCs with high solar-thermal efficiency.
To enhance the solar-thermal performance of PCCs, embedding carbon-based fillers with high thermal conductivity and good solar absorption has been regarded as an effective strategy[18,19]. Carbon-based fillers can be categorized into three configurations: 1D, 2D, and 3D structures. Carbon fiber (CF) and carbon nanotube (CNT) are typical 1D carbon-based fillers. Zhang et al.[20] embedded aligned continuous CFs into a PW-OBC blend to fabricate a PCC, which achieved a high directional thermal conductivity of 5.63 W·m-1·K-1 and a high solar-thermal efficiency (ηs-t) of 98.31% under 3 suns. Hu et al.[21] prepared a PCC by infiltrating paraffin with oriented CFs, obtaining a thermal conductivity of 5.21 W·m-1·K-1 with an enhanced ηs-t of 83.5% under 1 sun. Li et al.[22] developed a PCC with CNTs and carbon foam, achieving a thermal conductivity of 1.31 W·m-1·K-1 and a high ηs-t of 82.6 % under 3 suns. Graphene oxide (GO) and pressed EG are two typical 2D carbon-based fillers. Xia et al.[23] made a PCC by polymerizing GO and TiO2 onto a polyurethane porous medium, attaining a thermal conductivity of 0.7 W·m-1·K-1 and a ηs-t of 93.5% under 1 sun. Zhao et al.[24] fabricated a solid-solid PCC based on trimethylolethane and EG using a pressure-induced approach, which obtains a high lengthwise thermal conductivity of 12.82 W·m-1·K-1 and a ηs-t of 69.49% under 3 suns. Liu et al.[25] produced an EG-based PCC through melt blending and vacuum impregnation, reaching a thermal conductivity of 3.22 W·m-1·K-1 and a ηs-t of 80.81% under 1.2 suns. Carbon nanotube sponges/aerogels and graphene aerogels (GAs)/sponges can form continuous 3D thermally conductive networks within PCCs[26]. This 3D structure not only improves the thermal conductivity of the PCC but also allows a higher PCM proportion. For example, Zhou et al.[27] made a CNT-based PCC by vacuum impregnation, reaching a thermal conductivity of 0.433 W·m-1·K-1 and a ηs-t of 73.1% under 1.2 suns. Luo et al.[28] reported a PCC supported by a GA prepared via reduction and vacuum freeze-drying, which enables effective polyethylene glycol encapsulation. This PCC achieves a thermal conductivity of 0.348 W·m-1·K-1 and a ηs-t of 88.2% under 1.2 suns. Mu et al.[29] fabricated a PCC by grafting lauric acid onto the GA surface via esterification and reduction. At a GA mass fraction of 4.8%, the PCC obtains a thermal conductivity of 1.207 W·m-1·K-1 and a ηs-t of 80.6% under 1 sun.
The above results indicate that PCCs with 3D carbon-based fillers show lower thermal conductivity improvements compared with those employing 1D or 2D fillers. This phenomenon can be attributed to the relatively low compactness and low filling rate in the 3D porous structure, which limits thermal conductivity enhancement. For improving solar-thermal efficiency, 1D carbon-based fillers possess a superior ability compared to 2D or 3D fillers. The enhancing ability comes from the strong anisotropic heat conduction in 1D-based PCCs, which enhances heat transfer along the primary solar-thermal conversion direction while minimizing lateral heat loss[30], ultimately resulting in higher overall solar-thermal efficiency.
Therefore, to improve solar-thermal efficiency, it is necessary to optimize anisotropic thermal conductivities to achieve efficient heat transfer while minimizing heat loss. Although some PCCs with enhanced anisotropic thermal conductivities have been reported in recent years (Table S1), their relatively low thermal conductive anisotropy values result in relatively low solar-thermal efficiency. Therefore, developing a PCC that combines both high directional thermal conductivity and high thermal conductive anisotropy remains a challenge.
Herein, a laminated PCC was developed through pressure-induced assembly of alternately stacked PW-OBC and EG sheets to achieve both high lengthwise thermal conductivity and thermal conductive anisotropy. Firstly, a readily scalable fabrication method was developed via pressure-assisted lamination of PW-OBC with EG sheets. Then, the macroscopic morphology and microstructure of the PCC were characterized. Furthermore, its phase-change durability and transition characteristics were analyzed., Moreover, the anisotropic conductive characteristics for the PCC were measured. Lastly, the solar-thermal conversion and heat storage performance of the PCC were studied under varying solar irradiation conditions.
2. Materials and Methods
2.1 Materials
PW was supplied by Petro China Co., Ltd. (China), with a supplier-specified melting point of 48 °C. OBC with the grade of INFUSE 9530 was supplied by Dow Chemical (USA). It has a melt flow rate of 5 g/10 min under the conditions of 190 °C and 2.16 kg and a density of 0.887 g·cm-3. Qingdao Tengshengda Carbon Machinery Co., Ltd. (China) supplied raw graphite flakes with an average size of 300 μm. Yueyang Dongrun Chemical Co., Ltd. (China) provided carbon black with a purity of 99%.
2.2 Laminated PCC design and fabrication
OBC is expected to enhance the shape stability of PCCs by mitigating the PW leakage. Meanwhile, the thermal conductivity of the PCC can be improved by adding carbon-based materials into the PCM matrix. However, two significant challenges remain in achieving efficient solar-thermal energy storage in PCCs. The first is that insufficient internal thermal conductivity of PCCs can lead to surface overheating, resulting in substantial heat loss. The second is that enhancing thermal conductivity in non-solar-thermal conversion directions may promote undesirable heat dissipation to the surroundings. Hence, it is crucial to improve the anisotropic thermal conductivity of the PCC to facilitate rapid heat transfer within the material while reducing heat loss.
To achieve this goal, anisotropically conductive PCCs with parallel EG sheets using a lamination approach was designed and manufactured, as shown in Figure 1. First, PW and OBC were mixed thoroughly at a specific mass ratio at 170 °C for 60 minutes. Next, the liquid PW-OBC mixture was transferred into a mold and was allowed to solidify upon cooling to room temperature. The resulting solid mixture was demolded and then sliced into 2 × 2 cm2 thin sheets.

Figure 1. Fabrication sketch of the anisotropic conductive PCC with laminated structures. PW: paraffin wax; OBC: olefin block copolymer; PW-OBC: paraffin wax-olefin block copolymer; EG: expanded graphite; PCC: phase change composite.
EG was fabricated by thermally treating raw graphite flakes in a tube furnace at 750 °C for 15 minutes. Each single-layer PW-OBC sheet was then placed into a mold together with a corresponding mass of EG. They were compressed under 10 MPa to form single-layer PCC sheets. Finally, these prepared PCC sheets were arranged in a steel mold and pressed under 80 MPa to produce a square block.
PW contributes to the phase change enthalpy of the laminated PCCs. OBC acts as a polymer matrix to prevent PW leakage. The parallel EG sheets enhance directional thermal conductivity and the anisotropic degree of the blocks by forming oriented thermal conduction channels. Table 1 shows key characteristics of the laminated PCC samples.
| Item | OBC/wt.% | PW/wt.% | EG/wt.% | Density/kg·m-3 |
| PCC1 | 20 | 80 | 0 | 912.3 |
| PCC2 | 25 | 75 | 0 | 917.3 |
| PCC3 | 23.75 | 71.25 | 5 | 920.4 |
| PCC4 | 22.5 | 67.5 | 10 | 952.4 |
| PCC5 | 21.25 | 63.75 | 15 | 977.6 |
| PCC6 | 20 | 60 | 20 | 1,016.0 |
PCC: phase change composite; OBC: olefin block copolymer; PW: paraffin wax; EG: expanded graphite.
2.3 Characterization
The microstructures of the samples were examined using scanning electron microscopy (SEM, JSM-6360LW, JEOL). X-ray diffraction (XRD, D8 Advance, Bruker) was employed to determine the crystal structures at a scan rate of 5°·min-1. The phase change properties were examined with a differential scanning calorimeter (DSC 214, Netzsch) under heating/cooling rates of 10 °C·min-1, a nitrogen flow rate of 50 mL·min-1, and a holding time of 5 min. Thermal decomposition behaviors were investigated via thermogravimetric analysis (TG 209 F3, Netzsch) at a ramp rate of 10 °C·min-1 from 30 to 600 °C. The longitudinal and transverse thermal conductivities of all laminated PCC samples were determined using a transient plane source apparatus (DZDR-S, Nanjing Dazhan). Temperature contours at the side walls of the samples were captured using an infrared camera (MobIR Air, Guide). Spectral absorptances of different sample surfaces were obtained using an UV-Vis-NIR spectrophotometer (UV-3600i Plus, Shimadzu). The shape stability of the samples during thermal energy storage was assessed by heating them on an 80 °C heating plate, with the mass of each sample was measured using a precision balance (FA2204E, Xingyun) with an accuracy of 1 mg.
2.4 Solar-thermal experiment
The irradiation of the solar simulator (CEL-PF300-T8, CEAuLight) was calibrated using a flux meter (CEL-NP2000-2, CEAuLight). Then, laminated PCCs containing EG at different proportions were irradiated by the solar simulator under 1-3 sun irradiation (1 sun = 1 kW·m-2). Temperature evolution during thermal cycling was tested by a J-type thermocouple affixed to the sample’s bottom center and connected to a data acquisition system. After the testing, Eq. (1)[31,32] was employed to calculate the solar-thermal efficiency (ηs-t). Its maximum relative uncertainty is 4.44% according to the evaluation in Note S1 and Table S2.
where ΔH represents the enthalpy change of each sample during phase change (J·g-1); m denotes the sample mass (g); S stands for the sample’s surface area (m2); P refers to the intensity of simulated solar radiation (W·m-2); and Δt represents the illumination time during the phase transition process (s). Specifically, Δt is determined as the time interval between the initiation and end of the solar-induced phase change. The determination of these two critical time points was achieved through drawing tangent lines on the temperature-time curve.
3. Results and Discussion
3.1 Macroscopic morphology and microstructure
PCC6 was taken as an example to illustrate the macroscopic morphology and microstructures of the PCCs, as shown in Figure 2. Figure 2a shows the photograph of PCC6, revealing the composite featuring a clear layered architecture, with EG sheets neatly arranged along the longitudinal direction to create consecutive and oriented thermal conduction channels. It is worth noting that analogous layered structures are also visible in other PCC samples with varying EG contents (Figure S1).
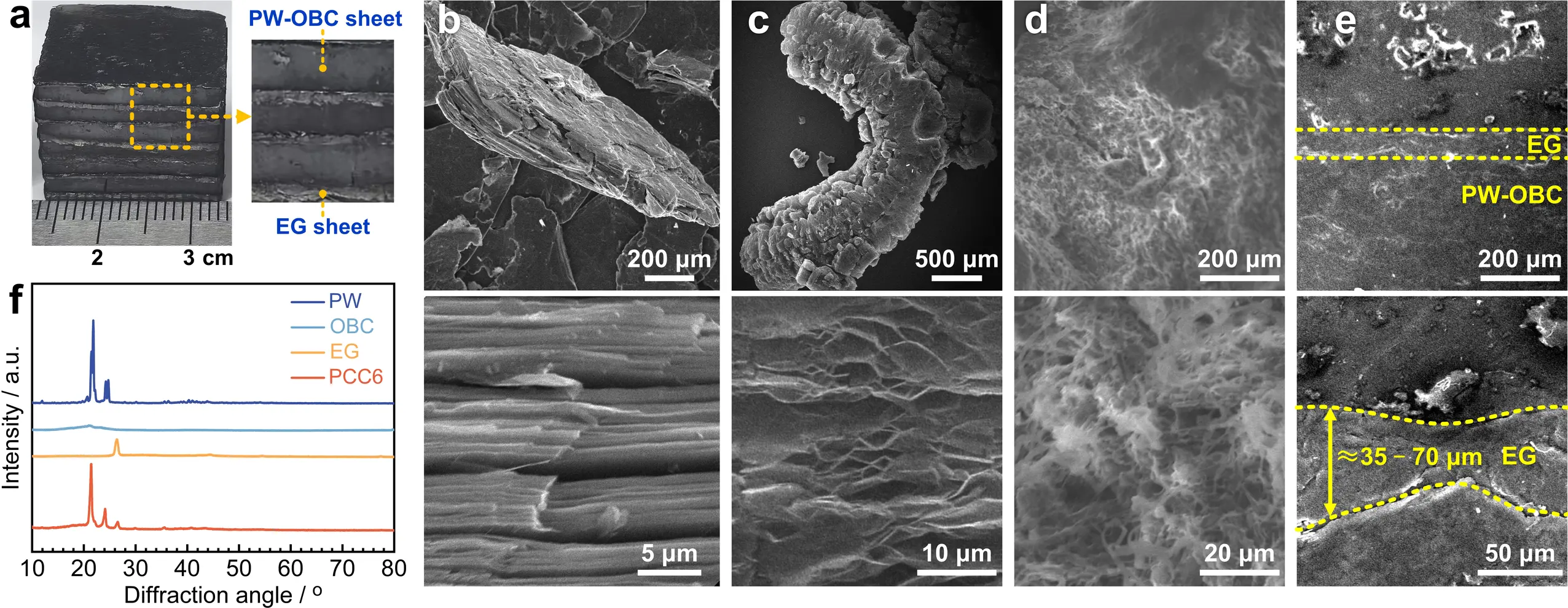
Figure 2. Macroscopic morphology and microstructure of the laminated PCC. (a) Photo of PCC6 showing its layered structure; (b) SEM images of raw graphite flakes; (c) SEM images of EG; (d) SEM images of PW-OBC sheet; (e) SEM images of the cross-section of PCC6; (f) XRD patterns of PW, OBC, EG, and PCC6. PW: paraffin wax; OBC: olefin block copolymer; PW-OBC: paraffin wax-olefin block copolymer; EG: expanded graphite; PCC: phase change composite; SEM: scanning electron microscopy; XRD: X-ray diffraction.
Figure 2b illustrates the raw graphite flakes, which show clear stratified structures in each flake. After being heated at 750 °C, worm-like EG was obtained (Figure 2c), naturally containing long-range thermal conduction channels. Furthermore, it can be seen in Figure 2d that OBC forms an interconnected porous framework within PCC6, where PW is homogeneously dispersed across the OBC soft segments, imparting superior shape stability to the composite. After compression molding, Figure 2e shows that the EG sheets with a thickness of approximately 40 μm, show almost no internal pores while maintaining consecutive conductive networks. Meanwhile, the close interfacial bonding between the EG sheets and adjacent PW-OBC layers reduces thermal contact resistance to a minimum, thereby enhancing the heat storage efficiency of the PW-OBC layers during solar-thermal energy conversion (Figure 2e).
To examine the crystalline structure of the PCCs, XRD analyses were conducted on PW, OBC, EG, and PCC6. As presented in Figure 2f, EG shows a single characteristic diffraction peak at roughly 26.4°, whereas OBC exhibits one peak at around 21.4°. In contrast, PW displays two distinct peaks at approximately 21.8° and 24.2°. The XRD pattern of PCC6 has three peaks at around 21.4°, 24.1°, and 26.4°, encompassing all the peaks of EG, OBC, and PW with no new peaks appearing. This indicates favorable chemical compatibility among the components in PCC6. It is also noteworthy that the intensity of these peaks in PCC6 is lower than in their respective individual phases. This phenomenon may stem from heightened structural disorder following material composite formation.
3.2 Phase-change durability and transition characteristics
PW easily leaks during heat storage due to the solid-liquid phase change. To mitigate this issue, OBC was employed as a polymer matrix to encapsulate and stabilize PW. The shape stability of the laminated PCCs was tested by placing PW and PCCs on filter papers (pore size ≈ 20 μm) and heating them on an 80 °C hot plate for 30 minutes. Leakage marks on the filter paper were visually assessed, while mass measurements were acquired at 5-minute intervals.
As shown in Figure S2, molten pure PW gradually forms leakage marks on the filter paper during heating, with the marks covering nearly the entire surface after 20 minutes, confirming its poor shape stability. PCC1 also showed leakage marks from molten PW, although these were evidently smaller than those from pure PW. After 30 minutes, PCC1 showed a mass loss rate of only 2.7% (Figure 3a, Table S3), as reported in Note S2, demonstrating the effectiveness of OBC in enhancing the shape stability of PCCs. Furthermore, PCC2 showed no visible leakage marks during heating, with a mass loss rate of just 0.2% after 30 minutes (Figure 3a, Figure S2). These results indicate that 25 wt.% OBC can effectively prevent PW leakage during solid-liquid phase change. Therefore, PW-OBC sheets with 25 wt.% OBC were selected for preparing PCC3-PCC6, which also showed no visible leakage marks, with mass loss rates below 0.3% after 30 minutes (Figure 3a, Table S3). In addition, the cyclic stability of PCC6 was evaluated over 1,000 heating-cooling cycles between 15 °C and 90 °C. The phase change enthalpy and DSC profiles of PCC6 were compared under different cycles (Figure 3b, Figure S3), which presented minimal variation, confirming the excellent long-term stability of the PCC, which is expected for practical applications.
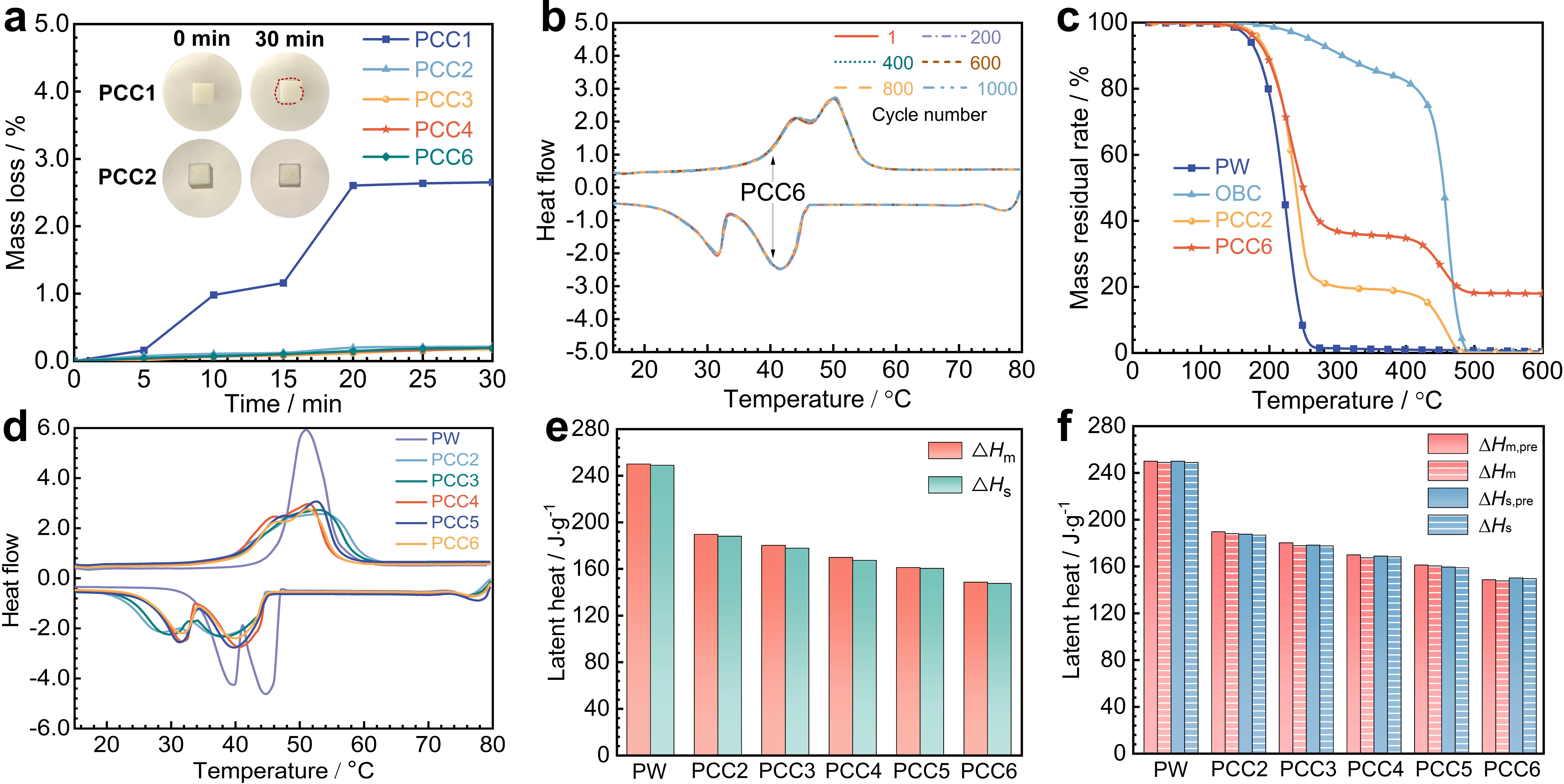
Figure 3. Phase-change durability and transition characteristics of laminated PCCs. (a) Temporal mass losses of typical PCC samples; (b) Stability of PCC6 over 1,000 thermal cycles; (c) Thermogravimetric decomposition profiles of typical samples; (d) Comparative DSC thermograms of PW versus PCC2-PCC6; (e) Measured melting (ΔHm) and solidification (ΔHs) enthalpies of samples; (f) Predicted (ΔHm,pre, ΔHs,pre) and measured (ΔHm, ΔHs) enthalpies of samples. PW: paraffin wax; OBC: olefin block copolymer; PCC: phase change composite; DSC: differential scanning calorimeter.
Next, TG analysis was used to further study the thermal stability of PCCs. As illustrated in Figure 3c, the TG curve of PW begins to drop at around 140 °C, indicating the initiation of PW decomposition, and falls to zero at around 260 °C, showing that PW has been fully decomposed. It is worth noting that OBC remains stable when the temperature is below 180 °C, and a plateau is observed between 330-410 °C, which corresponds to a residual mass of approximately 80%. Due to the presence of OBC, PCC2 shows a plateau at roughly 280-410 °C with a residual mass of approximately 20%, consistent with the mass proportion of OBC in PCC2. Similarly, PCC6 shows a plateau between 280-400 °C with a residual mass of approximately 36%, which is ascribed to the combined effects of undecomposed EG and OBC. These findings indicate that present PCCs can be stable below 140 °C.
Phase transition temperature and enthalpy are key parameters that define the heat storage performance of PCCs. Therefore, the phase transition behaviors of PCCs were analyzed via DSC thermograms (Figure 3d, Table S4). Similar trends are observed in the DSC thermograms of PCC2-PCC6. For PW, the melting and solidification temperatures (Tm, Ts) are 52.9 °C and 46.3 °C, respectively, while the corresponding values for PCC2 are 53.7 °C and 37.6 °C. These data indicate that adding OBC has little impact on the melting temperature of PCC but causes a significant decrease in its solidification temperature. It is inferred that this phenomenon is caused by physical cross-linking interactions between PW and OBC[33]. For PCC3-PCC6, the Tm and Ts ranges are 51.2-53.7 °C and 38.9-41.4 °C, respectively, showing slight variations compared with PCC2. This indicates that adding EG does not lead to significantly influence the phase transition behaviors of the PW-OBC composites.
Finally, as illustrated in Figure 3e, the tested data of melting enthalpies (ΔHm) and solidification enthalpies (ΔHs) of the PCCs decrease linearly with the decreasing PW mass proportion. Since PW, OBC, and EG are physically mixed, the phase change enthalpy of the PCC should theoretically scale as a linear function of PW mass proportion and can be predicted by using Eq. (2). As shown in Figure 3f and Table S4, comparisons of the predicted melting enthalpies (ΔHm,pre), ΔHm, predicted solidification enthalpies (ΔHs,pre), and the ΔHs of PW and PCCs demonstrate good agreement between theoretical predictions and experimental results.
where ΔHPW denotes the phase change enthalpy of pure PW (J·g-1) and φwt is the mass proportion of PW within PCC (wt.%).
3.3 Anisotropic conductive characteristics
Thermal conductivity critically governs heat transfer within PCCs during solar-thermal energy storage. Higher thermal conductivity enables photo-thermally converted heat at the PCC surface to transfer more rapidly into the interior, where it is stored by the PW-OBC mixture. However, high thermal conductivity in directions other than photo-thermal conversion can reduce solar-thermal efficiency, as it promotes heat loss to the environment. Therefore, optimizing thermal conductive anisotropy in PCCs is essential for increasing solar-thermal energy storage efficiency.
To improve anisotropic thermal conductivity, EG sheets were incorporated into the laminated PCCs as described in Section 2.2, forming a layered structure that constructs anisotropic thermal conduction paths. For insight into the mechanism of anisotropic heat conduction enhancement, parallel and series thermal transport models were established (Figure 4a, Figure S4, Note S3). In the parallel conduction model, thermal energy transfer occurs co-directionally with the EG conductive channels. This alignment minimizes the overall thermal resistance of the PCC, as quantified by the relation 1/Rpar = φv/REG + (1 - φv)/RPW-OBC, significantly boosting thermal charging and discharging rates. Conversely, in the series conduction model, heat flows orthogonally to the EG conductive channels, yielding the maximum total thermal resistance of Rser = φvREG + (1 - φv)RPW-OBC. This comparison indicates that controlling the orientation of the EG sheets within the PCC provides an effective means to improve its heat transfer ability.
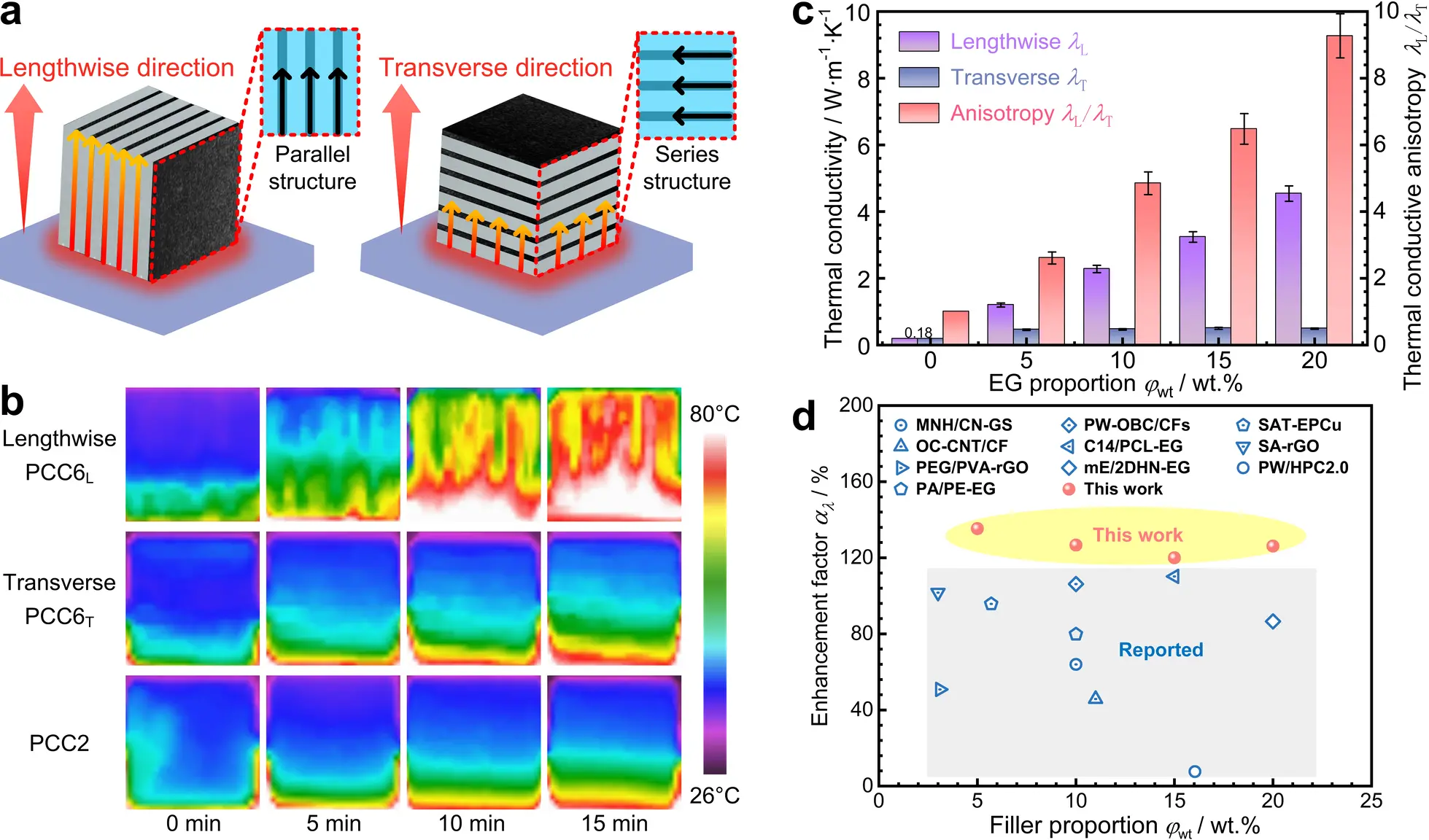
Figure 4. Anisotropic thermal conduction in the laminated PCCs. (a) Sketch of series and parallel thermal resistance models; (b) Lengthwise and transverse infrared thermograms of the heat transfer process for PCCs; (c) Directional thermal conductivities of PCCs across varying EG proportions; (d) Enhancing factors (αλ) of current PCCs and some reported samples[20,22,25,28,31,32,34-37]. EG: expanded graphite; PCC: phase change composite; MNH/CN-GS: melamine-naphthalene hybrid/carbon nanosphere-graphene sheet; PW-OBC: paraffin wax-olefin block copolymer; CF: carbon fiber; SAT: sodium acetate trihydrate; EPCu: modified expanded graphite; OC: octadecanol; CNT: carbon nanotube; C14: n-tetradecane; PCL: polycaprolactone; SA: salicylic acid; rGO: reduced graphene oxide; PEG: poly ethylene glycol; PVA: polyvinyl alcohol; mE: meso-erythritol; 2DHN: two-dimensional heterogeneous nanohybrids; PW: paraffin wax; HPC: hierarchical porous carbon; PA: polyamide; PE: polyethylene.
The ratio λL/λT represents the thermal conductive anisotropy, where λT and λL denote the transverse and longitudinal conductivities, respectively. Based on the series and parallel heat conduction models, its expression can be given by Eq. (3), based on Note S3. This equation indicates that the thermal conductive anisotropy initially rises and then decreases with the EG volume fraction in the PCC (φv), reaching its maximum at φ = 0.5. Meanwhile, its value is always higher than 1, indicating that the parallel structure along the lengthwise direction shows lower thermal resistance than the series structure along the transverse direction. Moreover, when φ is less than 0.5, the thermal conductive anisotropy increases with φ. Therefore, increasing the EG volume appropriately can effectively enhance λL/λT.
where λEG and λPW-OBC refer to the thermal conductivities of the EG and PW-OBC sheets, respectively.
To visually illustrate the anisotropic thermal conductivities of the PCCs, the lengthwise PCC6 (PCC6L), transverse PCC6 (PCC6T), and PCC2 were placed on an 80 °C constant-temperature heating plate. Their temperature distributions were captured using an infrared camera (Figure 4b). During heating, it can be observed that the upper region of the PCC2 without the EG sheet consistently shows a relatively low temperature, because its thermal conductivity is only 0.18 W·m-1·K-1 (Figure 4c).
PCC6 displays distinct temperature distributions along its lengthwise direction compared to its transverse direction (Figure 4b). Along the lengthwise direction, the excellent heat conduction ability of PCC6L promotes efficient upward heat transport through the structure. After 10 minutes, nearly half of the PCC6L side surface reaches 80 °C, with interlaced temperature stratification. Some regions near the tops of the EG sheets reach 80 °C, while the PW-OBC layer mostly exceeds 55 °C, which is above the phase transition point. However, in the transverse direction, even after 15 minutes, the upper region of the PCC6T remains at relatively low temperatures, with the heat from the heating plate accumulates near the contact surface. This stems from high transverse thermal resistance severely hindering heat transfer.
Then, the transverse (λT) and lengthwise (λL) thermal conductivities of the PCCs were tested and compared. Data in Figure 4c show that λL consistently exceeds λT across the entire range of EG mass proportion (φwt). With increasing EG proportion, λL rises sharply, while λT remains nearly unchanged, resulting in a consecutive increase in the thermal conductive anisotropy (λL/λT). When φwt increases from 0 wt.% to 20 wt.%, λL rises sharply from 0.18 W·m-1·K-1 to 4.54 W·m-1·K-1, whereas, λT only increases slightly from 0.18 W·m-1·K-1 to 0.49 W·m-1·K-1. As a result, the thermal conductive anisotropy rises sharply from 1.00 to 9.27, and this trend aligns well with the previously discussed thermal resistance models.
Finally, an enhancing factor for the thermal conductance (αλ), defined in Eq. (4)[30], serves to quantify the contribution of EG to the thermal conductance of the PCC (λPCC).
where φwt denotes the mass proportion of EG and λPW-OBC is the thermal conductance of the PW-OBC substrate.
The enhancing factors of present laminated PCCs were compared with those reported in previous studies (Figure 4d, Table S5). Most prior studies reported carbon-based PCCs show low αλ (below 120%). In contrast, present αλ reaches a maximum value of 135.33%, indicating that each 1 wt.% increase in EG proportion can enhance the lengthwise thermal conductance of the PCC by 135.33% relative to the pure PW. The satisfactory improvement in thermal conductance achieved by the present PCCs compared to existing PCCs may be attributed to two reasons. First, compared with using graphite flakes, graphene, or carbon nanotubes as thermal conductive fillers, the EG sheets fabricated by compression are more likely to form consecutive thermal conduction channels by connecting the worm-like layered EG (Figure 2c), thereby diminishing thermal resistance at the filler-PCM interface. Second, during lengthwise heat transfer, the parallel-structured thermal conduction network in the PCC exhibits lower thermal resistance (Figure 2e), leading to a superior heat transfer performance.
3.4 Solar-thermal conversion and heat storage performance
As discussed in the previous section, the laminated PCCs show excellent anisotropic thermal conductivity. In this section, their solar-thermal efficiency and energy storage performance are analyzed. As shown in Figure 5a, during the solar-thermal energy storage process, solar energy conversion mainly occurs on the irradiated top surface, while heat loss is primarily caused by convective and radiative transfer to the environment. To improve the solar-thermal efficiency, a direct solar-thermal efficiency energy storage device based on the developed PCC was developed (Figure 5a). Carbon black was applied to the top surface of the PCC to increase its solar absorptance, while a high-transparency glass cover and a thermal insulation layer were used to reduce radiative and conductive heat losses, respectively.

Figure 5. Solar-thermal conversion and heat storage performance. (a) Sketch of energy transfer processes in PCC6L with carbon black but without glass, and PCC6L with both carbon black and glass; (b) Solar absorption spectra of PCC6 with carbon black, PCC6, and PCC2; (c) Sketch of the solar-thermal testing setup; (d) Time-dependent temperature curves across EG mass proportions in PCCs at 1-sun irradiation; (e) Time-dependent temperature curves of PCC6L and PCC6T under different solar irradiations; (f) Solar-thermal efficiencies (ηs-t) for the glazed PCC6L, PCC6L, and PCC6T at variable irradiation; (g) Time-dependent temperature curves of PCC6L with/without a top glass cover at variable irradiation; (h) ηs-t data of PCC6L versus those of existing PCCs in literature[20,22,24,25,27-29,31,32,38,39]. PCC: phase change composite; EG: expanded graphite. OC: octadecanol; CF: carbon fiber; CNT: carbon nanotube; SAT: sodium acetate trihydrate; EPCu: modified expanded graphite; Ag-MWCNTs/PW@CNS: decorated carboxyl multi-wall carbon nanotubes/paraffin wax @Carbon nanotube sponge; PEG: poly ethylene glycol; PVA: polyvinyl alcohol; GO: graphene oxide; LA: lauric acid; GA: graphene aerogel; PDA: polydopamine; SiO2-MEPCM: silica hierarchical shell; mE: Meso-erythritol; 2DHN: two-dimensional heterogeneous nanohybrids; PAAS: polyamic acid salt; TME: trimethylolethane; PW: paraffin wax; OBC: olefin block copolymer; HPC: hierarchical porous carbon.
Initial analysis compares the solar absorption spectra of PCC6L with carbon black, PCC6L, and PCC2 in Figure 5b. It can be seen that PCC2 exhibits significant spectral fluctuation across the primary solar band (0.3-2.5 μm), with very low absorptance (~0.3) in the visible wavelength range.
After adding EG sheets into the PW-OBC composite, its solar-thermal efficiency conversion performance is significantly enhanced. PCC6L maintains 0.78-0.87 absorptance across the solar spectrum (0.3-2.5 μm), and its solar absorptance (Note S2) is up to 0.82, which is 0.43 higher than that of PCC2. Furthermore, after surface coating with carbon black, PCC6 exhibits further absorptance improvement across the full spectrum, reaching a high solar absorptance of 0.98. These results indicate that PCC6 with carbon black enables highly efficient full-spectrum solar-thermal conversion performance. These findings demonstrate that the carbon black coating can help the PCC achieve highly efficient full-spectrum solar energy capture.
A testing setup (Figure 5c, Figure S5) was constructed to evaluate the solar-thermal performance of the PCCs. To investigate the effects of thermal conductivity on PCC performance, PCC-based solar-thermal efficiency devices were exposed to 1-sun simulated solar irradiation, and their temperature-time profiles were recorded. As depicted in Figure 5d, the temperature of each PCC sample initially rises rapidly, then slows around 40 °C due to the phase transition. It surges again once the PW has fully melted, continuing until the solar simulator is switched off. These result demonstrate that the PCCs successfully achieve solar-thermal conversion, heat transfer, and storage. Furthermore, as the EG proportion increases, the heating curves of PCCs become steeper, and the phase change duration reduces. For instance, when the EG mass proportion increases from 0 wt.% to 20 wt.%, the phase change time decreases from 1,217 s to 559 s, resulting in an increase in solar-thermal efficiency (ηs-t) from 51.9% to 79.2% (Figure S6). These results indicate that high thermal conductivity enhances heat transport from top to bottom of the PCC, significantly improving solar-thermal efficiency.
To further explore the impacts of thermal conductive anisotropy and solar irradiation, PCC6L and PCC6T were tested under 1-3 suns. As shown in Figure 5e, for both PCC6L and PCC6T, the heating curves became gradually steeper with increasing solar irradiation, accompanied by shorter phase change durations and enhanced solar-thermal efficiency, which rises from 79.2% to 96.5% for PCC6L and from 66.2% to 81.3% for PCC6T (Figure 5f, Table S6, Table S7). Moreover, compared to PCC6T, PCC6L consistently exhibits a steeper heating curve, faster temperature rise rates, and shorter phase change durations, showing superior solar-thermal efficiency performance. For instance, under 3 suns, PCC6L achieves a 47 s reduction in phase change duration compared to PCC6T, with a solar-thermal efficiency improvement from 81.3% to 96.5%. To sum up, the anisotropic thermal conductivities of PCC6 (λL = 4.54 W·m-1·K-1, λT = 0.49 W·m-1·K-1) lead to different solar-thermal efficiency behaviors along its two normal directions.
Furthermore, the influence of a highly transparent glass cover on the solar-thermal efficiency performance was investigated. Figure 5g illustrates that, after covering with glass, the cooling curve of PCC6L becomes milder, indicating that the glass can effectively reduce thermal loss from the PCC. As a result, the phase change duration of PCC6L shortens under 1 sun, and its solar-thermal efficiency increases from 79.2% to 79.6% (Figure 5f, Table S6, Table S8). However, at high solar irradiation, the glass degrades the solar-thermal efficiency. For instance, the phase change duration of PCC6L with glass increases from 153 s to 156 s under 3 suns, and its solar-thermal efficiency declines from 96.5% to 94.6%. This phenomenon can be explained by the fact that, while the glass exhibits a transmittance of around 95%, the solar energy loss through the glass increases with increasing irradiation. At high solar irradiation, the glass-induced energy loss outweighs its benefit in reducing thermal loss, thereby reducing the solar-thermal efficiency.
Finally, PCC6L with carbon black was compared with some recently reported PCCs. Figure 5h and Table S9 indicate that the current design achieves higher solar-thermal efficiency across varying solar irradiation conditions. This could be attributed to its high lengthwise thermal conductivity, strong thermal anisotropy, and superior solar absorptance. These characteristics enable the solar-thermal efficiency conversion and rapidly transport heat for storage while minimizing heat loss to the environment.
4. Conclusions
This work develops an anisotropic laminated PCC through a simple method by incorporating parallel EG sheets into a PW-OBC mixture to overcome inherent limitations of PCMs, such as low heat transfer capacity, structural instability, and insufficient light absorption. The EG parallel sheets form consecutive heat transport channels within the PCC, yielding a notable lengthwise thermal conductivity of 4.54 W·m-1·K-1 and a high thermal conductive anisotropy of 9.27 at 20 wt.% EG proportion. The PCCs further show high latent heat (e.g., 148.4 J·g-1 for PCC6), coupled with exceptional thermal/cycling structural stability. Leveraging the synergistic advantages of anisotropic heat conduction of the PCC and excellent solar absorption introduced by carbon black, the PCC-based solar-thermal device attains 79.2%-96.5% solar-thermal efficiency under 1-3 suns irradiation, enabling effective solar energy capture and storage. In conclusion, this work provides a viable approach for fabricating high-performance PCCs for high-efficiency, low-to-medium temperature solar-thermal applications.
Supplementary materials
The supplementary material for this article is available at: Supplementary materials.
Acknowledgements
The authors are grateful for computational resources from the High Performance Computing Center of Central South University. Xiangkun Elvis Cao acknowledges financial support from the Schmidt Science Fellows Program by Schmidt Futures, in partnership with the Rhodes Trust.
Authors contribution
Huang H, Yang X: Investigation, visualization, data curation, writing-original draft.
Qiu Y: Conceptualization, methodology, investigation, writing-review & editing, supervision, funding acquisition.
Cao XE: Methodology, investigation, resources, writing-review & editing.
Liu L: Investigation, formal analysis;
Li C, Chen X, Yan H: Investigation, resources.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (No. 52476235), the Natural Science Foundation of Hunan Province (No. 2024JJ4059), Changsha Outstanding Innovative Youth Training Program (No. kq2306010), and the Central South University Innovation-Driven Research Programme (No. 2023CXQD055).
Copyright
© The Author(s) 2025.
References
-
1. Allen MR, Frame DJ, Friedlingstein P, Gillett NP, Grassi G, Gregory JM, et al. Geological Net Zero and the need for disaggregated accounting for carbon sinks. Nature. 2025;638(8050):343-350.[DOI]
-
2. Jiao K, Lu L, Wen T, Wang Q. Endowing photothermal materials with latent heat storage: A state-of-art review on photothermal PCMs. Chem Eng J. 2024;500(6):156498.[DOI]
-
3. Saher S, Johnston S, Esther-Kelvin R, Pringle JM, MacFarlane DR, Matuszek K. Trimodal thermal energy storage material for renewable energy applications. Nature. 2024;636(8043):622-626.[DOI]
-
4. He Y-L, Qiu Y, Tao YB, Wang K. Principles, technologies and numerical methods of solar thermal power. Beijing: Science Press; 2023.
-
5. Xu Q, Liu X, Luo Q, Yao H, Tian Y, Wang J, et al. Bioinspired spectrally selective phase-change composites for enhanced solar thermal energy storage. Adv Funct Mater. 2025;35(1):2412066.[DOI]
-
6. Yuan Z, Du C, Qiao L, Qiao J, Ye T, Huang A, et al. Solid-liquid phase change materials for solar-driven interfacial evaporation: Principles, design optimization, and emerging advances in sustainable seawater desalination. Chem Eng J. 2025;516:164024.[DOI]
-
7. Fu K, Mo S, Li Q, Zhou Z, Jia L, Du Y, et al. Preparation and thermal performance of a novel 1,10-decanediol -paraffin/expanded graphite composite phase change material for solar thermal utilization. Sol Energy Mater Sol Cells. 2025;289:113684.[DOI]
-
8. Li S, Jiao X, Chai L, Su Z, Yang Y, Zhao Y, et al. Assembling halloysite nanotubes in nickel foam with silica fibers as scaffold for efficiently encapsulating phase change materials towards solar-thermal-electric energy conversion and management. Chem Eng J. 2025;510:161681.[DOI]
-
9. Lin W, Lai J, Xie K, Liu D, Wu K, Fu Q. D-mannitol/graphene phase-change composites with structured conformation and thermal pathways allow durable solar-thermal-electric conversion and electricity output. ACS Appl Mater Interfaces. 2022;14(34):38981-38989.[DOI]
-
10. Zhai X, Xu Z, Zhang W, Zhang Q, Yang X, Qu J, et al. Phase change thermal energy storage: Materials and heat transfer enhancement methods. J Energy Storage. 2025;123(10):116778.[DOI]
-
11. Wu MQ, Wu S, Cai YF, Wang RZ, Li TX. Form-stable phase change composites: Preparation, performance, and applications for thermal energy conversion, storage and management. Energy Storage Mater. 2021;42:380-417.[DOI]
-
12. Baniasadi H, Fathi Z, Abidnejad R, Silva PES, Bordoloi S, Vapaavuori J, et al. Biochar-infused cellulose foams with PEG-based phase change materials for enhanced thermal energy storage and photothermal performance. Carbohydr Polym. 2025;123999.[DOI]
-
13. Wu S, Li T, Wu M, Xu J, Hu Y, Chao J, et al. Highly thermally conductive and flexible phase change composites enabled by polymer/graphite nanoplatelet-based dual networks for efficient thermal management. J Mater Chem A. 2020;8(38):20011-20020.[DOI]
-
14. Zhu Y, Qin Y, Liang S, Chen K, Tian C, Wang J, et al. Graphene/SiO2/n-octadecane nanoencapsulated phase change material with flower like morphology, high thermal conductivity, and suppressed supercooling. Appl Energy. 2019;250(6):98-108.[DOI]
-
15. Luo G, Liu H, Han N, Zhang X-x. Efficient encapsulation of organic phase change materials through Diels-Alder reaction and in situ assembly for excellent photothermal conversion and energy storage. Chem Eng J. 2025;512:162411.[DOI]
-
16. Sun L, Huang Y, Chen W, Jiang X, Hu X. Chemically cross-linked flexible polymer phase change material with self-healing, self-adaptive, and shape-memory properties for thermal interface applications. J Colloid Interface Sci. 2025;695(23):137831.[DOI]
-
17. Jing Y, Zhao Z, Cao X, Sun Q, Yuan Y, Li T. Ultraflexible, cost-effective and scalable polymer-based phase change composites via chemical cross-linking for wearable thermal management. Nat Commun. 2023;14(1):8060.[DOI]
-
18. Mishra AK, Lahiri BB, Philip J. Carbon black nano particle loaded lauric acid-based form-stable phase change material with enhanced thermal conductivity and photo-thermal conversion for thermal energy storage. Energy. 2020;191(1):116572.[DOI]
-
19. Tang Z, Gao H, Chen X, Zhang Y, Li A, Wang G. Advanced multifunctional composite phase change materials based on photo-responsive materials. Nano Energy. 2021;80:105454.[DOI]
-
20. Zhang P, Qiu Y, Ye C, Li Q. Anisotropically conductive phase change composites enabled by aligned continuous carbon fibers for full-spectrum solar thermal energy harvesting. Chem Eng J. 2023;461:141940.[DOI]
-
21. Hu J, Yao Z, Chen A, Xiao C, Zhang G, Yang X. High thermal-conductive phase change material by carbon fiber orientation for thermal management and energy conversion application. Appl Ther Eng. 2025;265(5):125566.[DOI]
-
22. Li SY, Yan T, Huo Y-J, Pan WG. Carbon nanotube/carbon foam thermal-bridge enhancing solar energy conversion and storage of phase change materials. Mater Today Sustain. 2024;28:100986.[DOI]
-
23. Xia T, Wu B, Zhang H, Xu F, Sun L, Lin X, et al. Titanium dioxide/graphene oxide synergetic reinforced composite phase change materials with excellent thermal energy storage and photo-thermal performances. J Mater Res Technol. 2024;32:4019-4027.[DOI]
-
24. Zhao Y, Zhang P, Qiu Y, Li Q, Yan H, Wang Z, et al. Highly conductive solid-solid phase change composites and devices enhanced by aligned graphite networks for solar/electro-thermal energy storage. DeCarbon. 2024;5:100051[DOI]
-
25. Liu P, Wang C, Cheng X, Wang S, Wang Z. Preparation and photothermal investigation of PDA and CuNPs coupled modified expanded graphite with sodium acetate trihydrate composite phase change materials. Sol Energy. 2025;293(7):113478.[DOI]
-
26. Tong X, Li N, Zeng M, Wang Q. Organic phase change materials confined in carbon-based materials for thermal properties enhancement: Recent advancement and challenges. Renew Sustain Energy Rev. 2019;108:398-422.[DOI]
-
27. Zhou L, Wang X, Wu Q, Ni Z, Zhou K, Wen C, et al. Carbon nanotube sponge encapsulated Ag-MWCNTs/PW composite phase change materials with enhanced thermal conductivity, high solar-/electric-thermal energy conversion and storage. J Energy Storage. 2024;84(1):110925.[DOI]
-
28. Luo L, Luo W, Chen W, Hu X, Ma Y, Xiao S, et al. Form-stable phase change materials based on graphene-doped PVA aerogel achieving effective solar energy photothermal conversion and storage. Sol Energy. 2023;255:146-156.[DOI]
-
30. Zhang P, Wang Y, Qiu Y, Yan H, Wang Z, Li Q. Novel composite phase change materials supported by oriented carbon fibers for solar thermal energy conversion and storage. Appl Energy. 2024;358:122546.[DOI]
-
31. Sun K, Kou Y, Zhang Y, Liu T, Shi Q. Photo-triggered hierarchical porous carbon-Based composite phase-change materials with superior thermal energy conversion capacity. ACS Sustain Chem Eng. 2020;8(8):3445-3453.[DOI]
-
32. Pan C, He P, Chen N, Wu J, Shi E, Wang A, et al. Highly thermally conductive composite phase-change materials doped with two-dimensional heterogeneous nanohybrids for photo/electro-thermal energy storage. J Energy Storage. 2023;57(21):106225.[DOI]
-
33. Sun Y, Zhang N, Sun Q, Cao X, Shao X, Yuan Y. A novel form-stable phase change material based on elastomeric copolymer and carbon nanotubes with photo-thermal conversion performance. J Energy Storage. 2023;63:107043.[DOI]
-
34. Wei Y, Li J, Sun F, Wu J, Zhao L. Leakage-proof phase change composites supported by biomass carbon aerogels from succulents. Green Chem. 2018;20(8):1858-1865.[DOI]
-
35. Zhang W, Ling Z, Fang X, Zhang Z. Anisotropically conductive Mg(NO3)2·6H2O/g-C3N4-graphite sheet phase change material for enhanced photo-thermal storage. Chem Eng J. 2022;430(7):132997.[DOI]
-
36. Xu C, Shen L, Wen Z, Zhou R, Hao M, Jiang J. Synergistic enhancement of shape stability and thermal properties of phase change materials by MOF/EG composite carriers. Mater Lett. 2025;395:138674.[DOI]
-
37. Zeng C, Shu Q, Li W, Qin X. Enhancing thermal performance of low-temperature phase change materials based on tetradecane. Energy Build. 2025;345:116112.[DOI]
-
38. Sun Z, Shi T, Wang Y, Li J, Liu H, Wang X. Hierarchical microencapsulation of phase change material with carbon-nanotubes/polydopamine/silica shell for synergistic enhancement of solar photothermal conversion and storage. Sol Energy Mater Sol Cells. 2022;236:111539.
-
39. Zhang Q, Dai Z, Gu B, Zhao D. Thermally conductive phase change composites for efficient medium-temperature solar thermal storage. J Energy Storage. 2025;113:115687.[DOI]
Copyright
© The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s Note
Share And Cite