Table of Contents
Enantioselectivity synthesis of isoquinolin-1-one derivatives with C–N axial chirality via cobalt-catalyzed oxidative formal (4+2) cycloaddition: Light or not
Developing mild and sustainable strategies for the synthesis of complex molecules is a pivotal yet challenging goal in modern synthesis. While cobalt catalysis offers a sustainable alternative to noble metals, achieving such transformations at room temperature ...
More.Developing mild and sustainable strategies for the synthesis of complex molecules is a pivotal yet challenging goal in modern synthesis. While cobalt catalysis offers a sustainable alternative to noble metals, achieving such transformations at room temperature remains elusive. Herein, we report a cobalt-catalyzed aerobic oxidative asymmetric formal cycloaddition involving C(sp2)–H bond activation of benzamides with unactivated alkynes at room temperature using air as the oxidant. For challenging low-activity substrates, reaction efficiency is enhanced via a photoinduced catalytic cycle. Mechanistic and substrate scope studies indicate that substrate reactivity is influenced by electronic and steric effects.
Less.Liang-Neng Wang, ... Liang-Qiu Lu
DOI:https://doi.org/10.70401/cc.2026.0012 - January 28, 2026
Development of a scalable iridium-catalyzed asymmetric hydrogenation process for synthesis of chiral 2-methyl-1,2,3,4-tetrahydroquinoline
Asymmetric hydrogenation is an efficient tool for rapid synthesis of a diverse range of chiral compounds with high yields and excellent enantioselectivities. Chiral 2-methyl-1,2,3,4-tetrahydro-quinoline is a valuable building block for organic synthesis ...
More.Asymmetric hydrogenation is an efficient tool for rapid synthesis of a diverse range of chiral compounds with high yields and excellent enantioselectivities. Chiral 2-methyl-1,2,3,4-tetrahydro-quinoline is a valuable building block for organic synthesis and has been widely used in the synthesis of bioactive molecules and chiral ligands. Herein, we report an improved iridium-catalyzed asymmetric hydrogenation of 2-methylquinoline on a hundred-gram scale for efficient synthesis of chiral 2-methyl-1,2,3,4-tetrahydroquinoline with up to 91.4% ee value and an 80,000 turnover number. Optically pure 2-methyl-1,2,3,4-tetrahydroquinoline could be obtained in high yields through recrystallization or chemical resolution with the tartaric acid derivative (L)-DMTA.
Less.Huan Jing, ... Yong-Gui Zhou
DOI:https://doi.org/10.70401/cc.2026.0010 - January 08, 2026
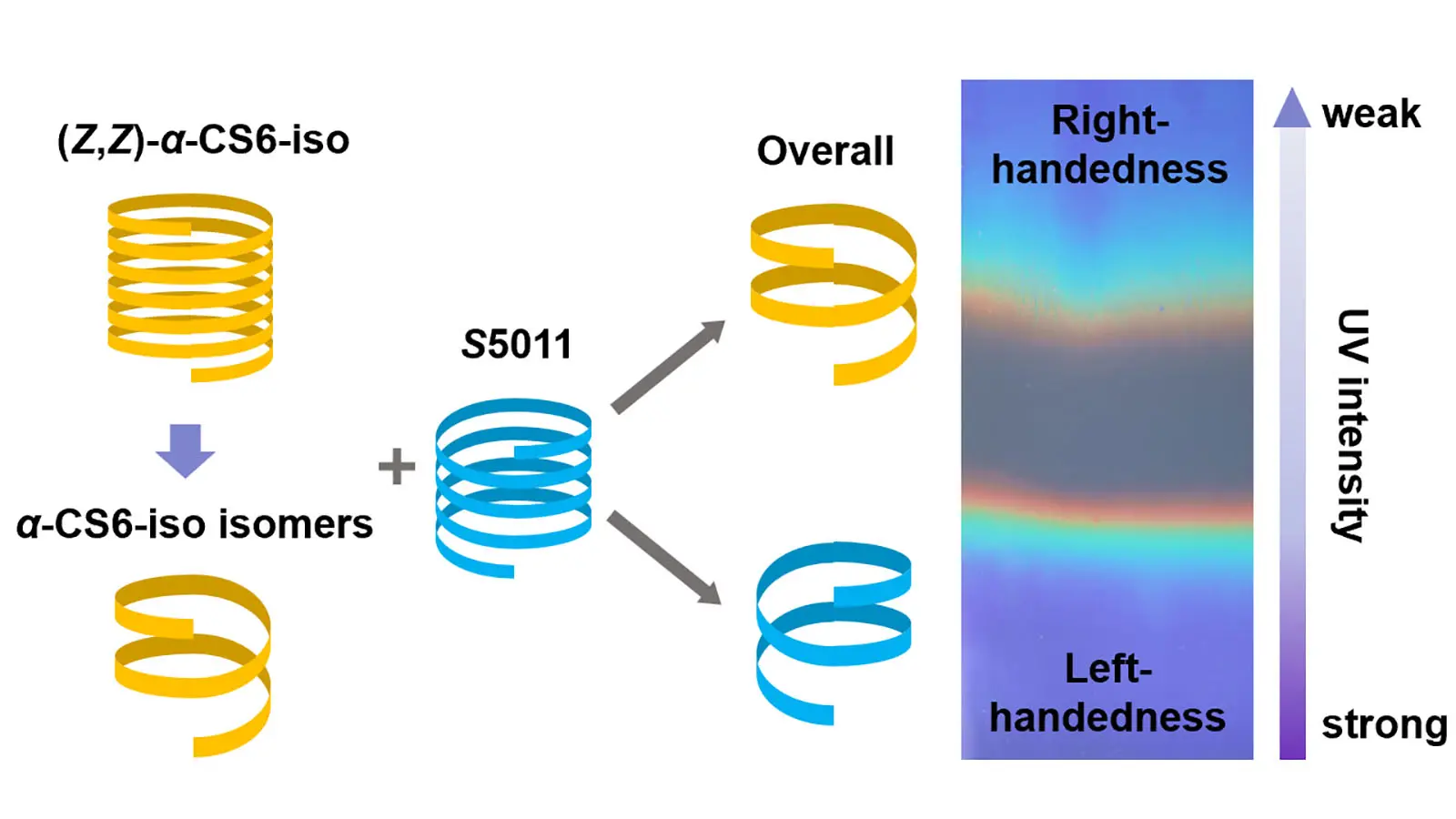
Patterned cholesteric liquid crystal polymer network film with precisely controlled structural colors prepared using a handedness invertible photochromic cholesteric liquid crystal mixture
Cholesteric liquid crystal polymer network (CLCN) patterns composed of oppositely handed helical structures are attractive for anti-counterfeiting. Different areas can be observed under the circularly polarized light with opposite handedness. However, ...
More.Cholesteric liquid crystal polymer network (CLCN) patterns composed of oppositely handed helical structures are attractive for anti-counterfeiting. Different areas can be observed under the circularly polarized light with opposite handedness. However, achieving precise control over the reflection band wavelengths of CLCN patterns using photochromic cholesteric liquid crystals (CLCs) remains a significant challenge. Herein, we synthesized a chiral cyanostilbene derivative that demonstrated significant modulation of its helical twisting power upon 365 nm ultraviolet (UV) irradiation. When incorporated into CLC mixtures, this additive enabled precise control over both the handedness and reflection wavelength of the resulting CLCN patterns within seconds under UV exposure. The system allowed for the preparation of colorful CLCN patterns through in-situ photopolymerization. These findings demonstrate that the developed CLC mixtures are well-suited for high-throughput fabrication of CLCN patterns on industrial coating lines.
Less.Xingchen Liu, ... Yonggang Yang
DOI:https://doi.org/10.70401/cc.2025.0009 - December 31, 2025
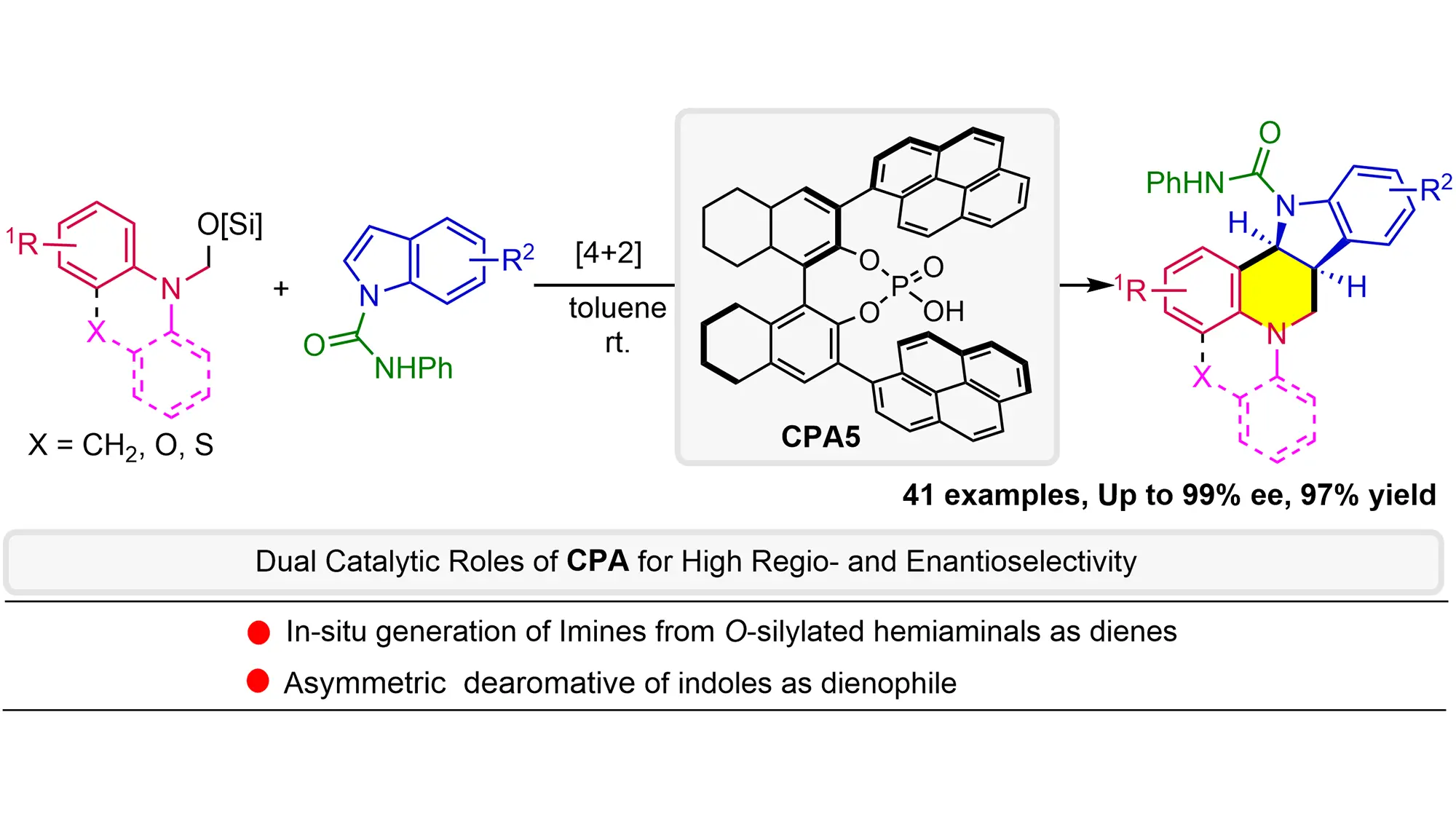
Catalytic asymmetric dearomative formal [4+2] annulation of indoles with O-Silylated hemiaminals as dienes: The dual role of chiral phosphoric acid
O-Silylated hemiaminals are utilized as elegant imine precursors in the formal asymmetric [4+2] annulation of indoles for the first time, wherein chiral phosphoric acid (CPA) acts (1) as a Brønsted acid catalyst to facilitate methanimine formation ...
More.O-Silylated hemiaminals are utilized as elegant imine precursors in the formal asymmetric [4+2] annulation of indoles for the first time, wherein chiral phosphoric acid (CPA) acts (1) as a Brønsted acid catalyst to facilitate methanimine formation under mild conditions and then (2) as an anion-binding catalyst for dearomative annulation. This methodology exhibits a broad substrate scope with remarkable functional group tolerance and enantioselectivity (up to 97% yield and 99% ee), providing straightforward access to the challenging indoline-fused tetrahydroquinolines bearing multiple stereogenic centers. Mechanistic studies reveal the critical role of PhNHCO- groups in enhancing both reactivity and enantioselectivity, probably due to non-covalent interactions with CPA. The kinetic isotope effects experiment and negative linear Hammett correlation suggest a concerted process.
Less.Nan-Fang Mo, ... Zheng-Hui Guan
DOI:https://doi.org/10.70401/cc.2025.0008 - December 26, 2025
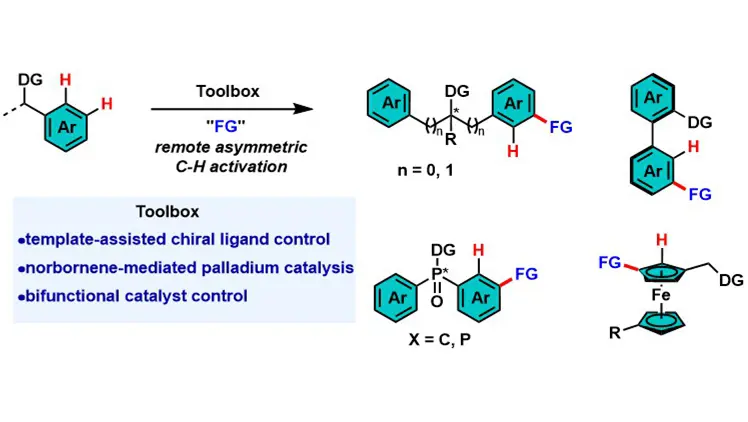
Transition metal-catalyzed remote asymmetric C–H activation of arenes
Transition metal-catalyzed asymmetric C–H activation is vital for chiral molecule synthesis but faces challenges in remote C–H functionalization due to traditional metallacycle constraints and difficulties in long-range chiral recognition. This review ...
More.Transition metal-catalyzed asymmetric C–H activation is vital for chiral molecule synthesis but faces challenges in remote C–H functionalization due to traditional metallacycle constraints and difficulties in long-range chiral recognition. This review summarizes three core strategies to address these issues: template-assisted chiral ligand control, norbornene-mediated palladium catalysis, and bifunctional catalyst control. These strategies achieve high enantioselectivity for diverse chiral architectures. Future directions include expanding to para-C–H bonds of arenes and aliphatic C–H bonds, developing robust chiral mediators/ligands, and applying the methodology to natural products and complex materials.
Less.Lili Chen, Senmiao Xu
DOI:https://doi.org/10.70401/cc.2025.0006 - December 16, 2025